Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
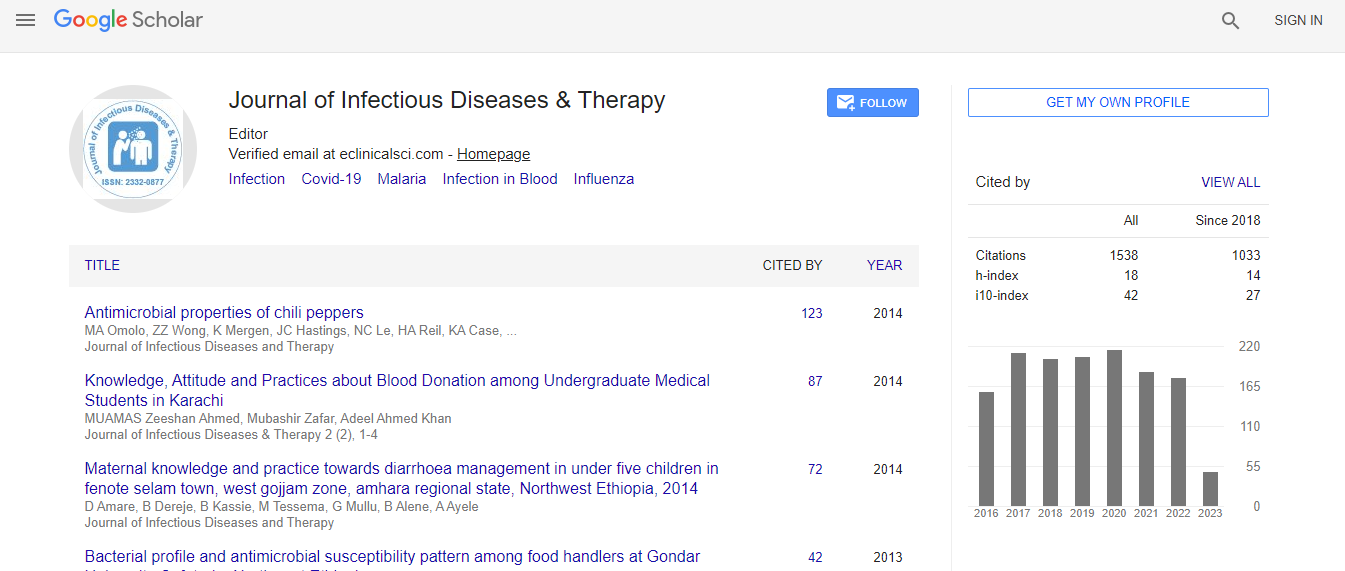
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Quantification of the haemagglutinin in monovalent influenza vaccines by a latex agglutination assay (LAA) as an alternative to the single radial immunodiffusion (SRID) assay
2nd International Conference on Influenza
Sophie Buffin
Sanofi Pasteur, France
Posters & Accepted Abstracts: J Infect Dis Ther
Abstract
To formulate inactivated influenza vaccines, the concentration of haemagglutinin (HA) must be accurately determined. The standard test currently used to measure HA in influenza vaccines is the single radial immunodiffusion (SRID) assay. The SRID assay is a cumbersome technique presenting a number of drawbacks such as low sensitivity and interference by some adjuvants. We developed a very simple, sensitive and rapid alternative HA assay using latex agglutination. The LAA uses the Spherotest├?┬« technology, which is based on the agglutination of HA-specific immunoglobulin-coated latex beads, which bind to the HA. The amount of HA in a sample can then be calculated from the level of bead agglutination by a simple absorbance measurement. A standard curve is generated using serially diluted HA reference protein. The results show that for monovalent A/H5N1 and A/H1N1 vaccines, the LAA demonstrated equivalent linearity, accuracy and precision as compared to the SRID assay. Moreover, unlike the SRID assay, LAA enables HA quantification in AlOOH-adjuvanted vaccines and in emulsion-based adjuvanted vaccines without interference. In addition, LAA was found to be more simple, rapid and sensitive than SRID. In conclusion, LAA may be useful to rapidly and accurately quantify the influenza HA protein in monovalent vaccines, especially in those formulated with low amounts of HA in the presence of an adjuvant.Biography
Sophie Buffin joined the Research department of Sanofi Pasteur in 2005 with a Master’s degree. She is currently a Ph.D. student at the University of Lyon.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi