Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
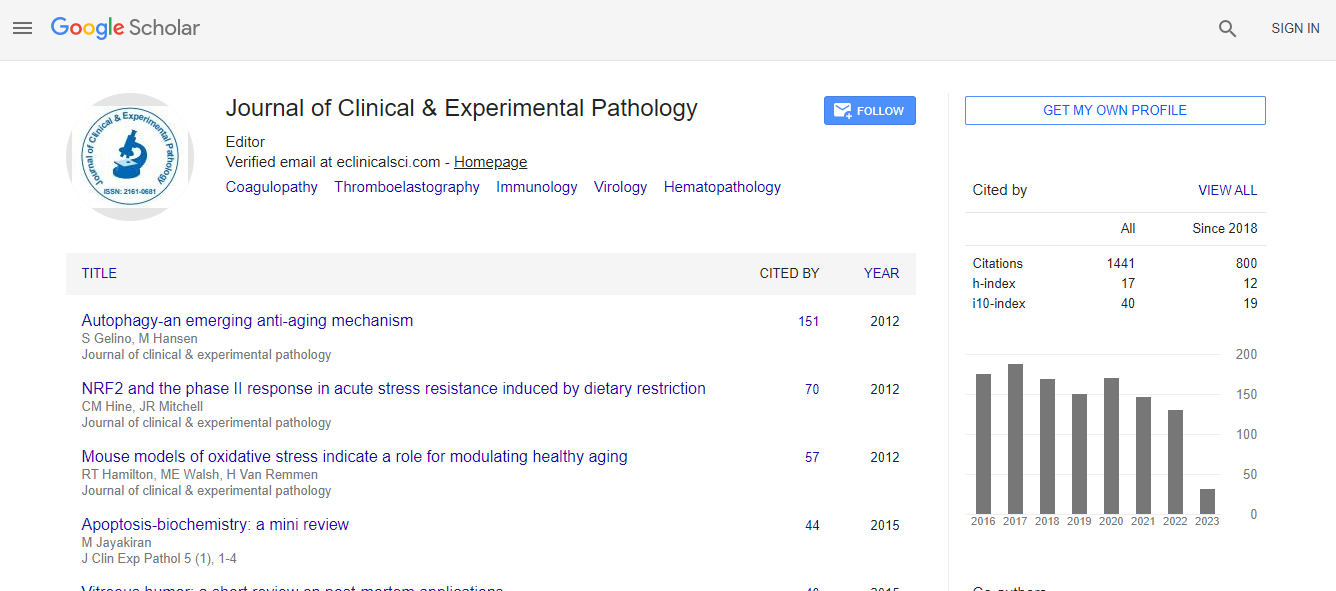
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Preclinical development of animal models for rheumatoid arthritis that is more clinically relevant and more focused on targeted therapeutics
6th European Pathology Congress
Kathy Kane
PharmaLegacy Laboratories, China
ScientificTracks Abstracts: J Clin Exp Pathol
Abstract
In the past two decades, antibody drugs have achieved great success in the treatment of many human diseases, due to their superior efficacies and pharmacokinetic characteristics. Antibody drugs bind to human disease target molecules with great specificity, and do not cross-react with most rodent targets. This has posted a big challenge to the traditional preclinical efficacy evaluation systems, which are mainly based on rodent models. However, antibody drugs can cross-react with most target molecules in Non-human Primate (NHP) models due to the high homology of these NHP targets with their human counterparts. This makes NHP models an ideal system for preclinical efficacy evaluation of antibody therapies. Since 2009, PharmaLegacy has been dedicated to developing a preclinical evaluation system for antibody therapies based on NHP. Now, PharmaLegacy has established a range of NHP models in inflammation/autoimmune and bone disease areas and has successfully evaluated many candidate mAb drugs binding to important disease targets like TNF├?┬▒/IL-6/IL-1├?┬▓ and RANKL/Sclerostin. Our experiences have fully demonstrated that NHP models form an ideal preclinical efficacy evaluation system for antibody drugs.Biography
Kathy Kane is the Director of Business Development for PharmaLegacy. She is a Management Executive with over 14 years’ experience in the Biotech Industry in Sales Management and Business Development.
Email: kathy.Kane@pharmalegacy.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi