Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
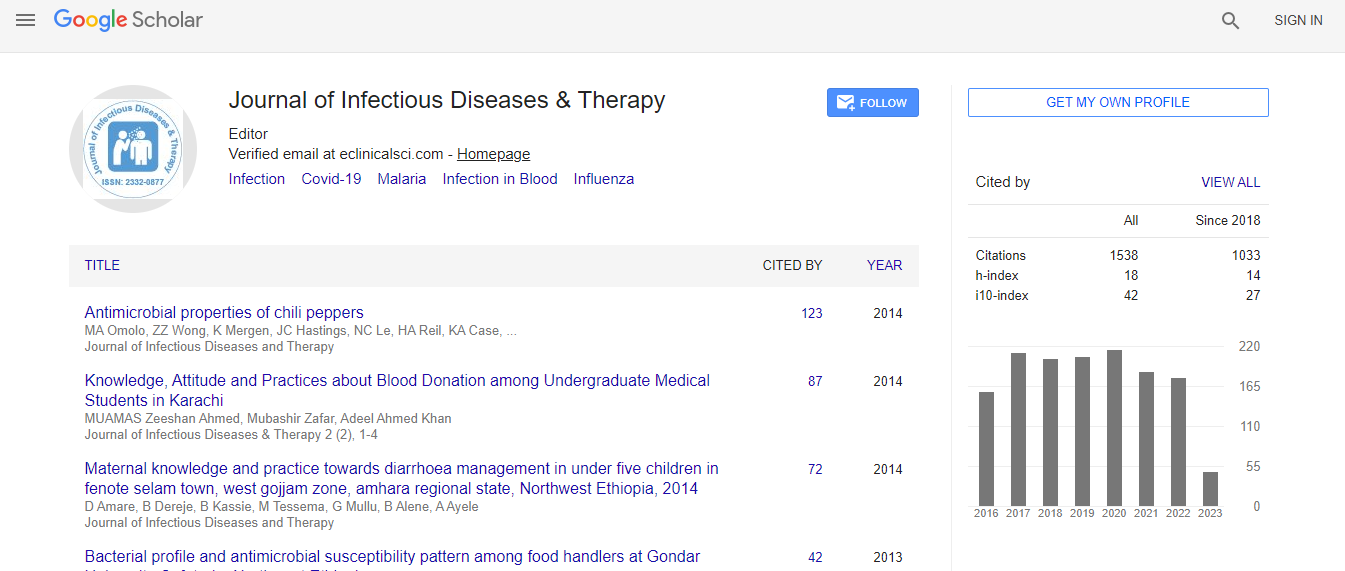
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Pharmacokinetics of ceftiofur after single intravenous and intramuscular injections in rabbits
World Congress on Infectious Diseases
Kamil UNEY1, Feray ALTAN2, Ayse ER1, Enver YAZAR1 and Muammer ELMAS1
Posters-Accepted Abstracts: J Infect Dis Ther
Abstract
Ceftiofur is a broad spectrum third generation cephalosporin antibiotic developed especially for use in animals. The aim
of present study is to determine the pharmacokinetics of ceftiofur and its metabolites after single intravenous (IV) and
intramuscular (IM) injections at a dose of 2.2 mg/kg BW in rabbits. Six healthy, male, 7-9 months, 3.14±0.28 kg rabbits were
used for the study. In pharmacokinetic study, the crossover design was performed. The withdrawal interval between the phases
of the study was 15 days. Ceftiofur sodium was administered by IV bolus and IM injections at the dosage of 2.2 mg/kg BW to
each rabbit. Plasma concentrations of desfuroylceftiofur were determined using the high-performance liquid chromatography.
Plasma concentration–time curve of desfuroylceftiofur was best fitted to a two-compartment open model. Following IV
injection, the major pharmacokinetic parameters (mean±SD) were distribution half-life (t1/2α) 0.34±0.07 h, elimination halflife
(t1/2β) 2.75±0.59 h, volume of distribution at steady state (Vdss) 260±71 mL/kg, area under the plasma concentrationtime
curve (AUC) 20.47±1.86 μg.h/mL, total body clearance (ClT) 108±10 mL.h/kg. After IM administration, the principal
pharmacokinetic parameters (mean±SD) were absorption half-life 0.09±0.03 h, peak plasma concentration 9.94±1.35 μg/
mL, time to peak concentration 0.25 h, t1/2α 0.31±0.16 h, t1/2β 2.84±0.68 h, AUC 20.11±2.49 μg.h/mL. The bioavailability
after IM injection was 98±4.4%. Results indicated that ceftiofur was absorbed quickly and excellent bioavailability after IM
administration. Single IV and IM injections of ceftiofur at a dose of 2.2 mg/kg may be effective to maintain the minimum
inhibitory concentration (MIC) upto 12h in rabbits against susceptible pathogens with MIC≤1 μg/ml.
Biography
Kamil UNEY (PhD, Assoc. Prof.) is currently working in Faculty of Veterinary Medicine, Selcuk University, Turkey, as teaching staff and principal investigator in
Department of Pharmacology and Toxicology since 2003. He graduated from Selcuk University, Faculty of Veterinary Medicine (2001). He received Pharmacology
and Toxicology degree from Selcuk University, Graduate School of Health Sciences (2007). His current research projects are studies including pharmacokinetics,
therapeutic drug monitoring, the development and validation of method in drug analysis and producing of quality control sera. He has also studies in the drug
metabolism, transporter proteins and the use of probe drugs in animals.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi