Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
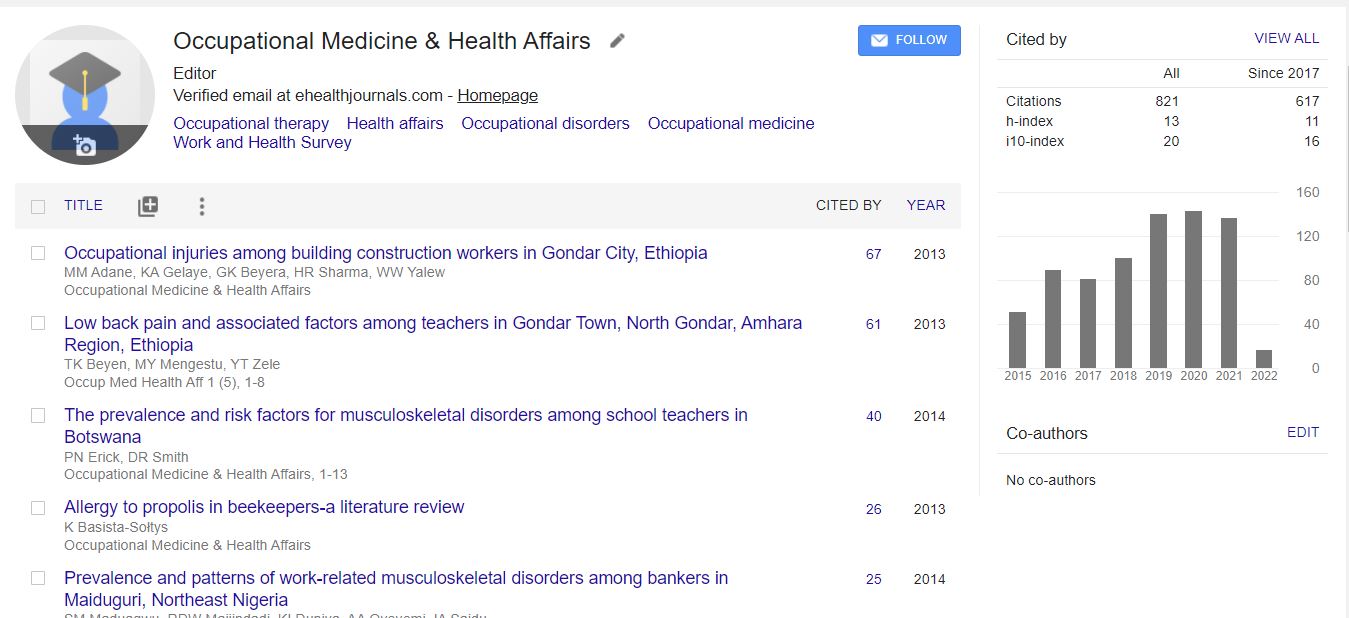
Google Scholar citation report
Citations : 1907
Occupational Medicine & Health Affairs received 1907 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Academic Keys
- China National Knowledge Infrastructure (CNKI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Patient safety: Assurance through accreditation?
12th World Congress on Industrial Health, Healthcare and Medical Tourism
Sweeney John Sweeney Y
Health Care Informed, Ireland
Keynote: Occup Med Health Aff
Abstract
Fundamentally the process of accreditation has not changed since its emergence in the 1960�s in the US, that being a �StopStart� process where organizations pour enormous resources into on-site surveys, only to tread water between these visits and allow the implementation requirements to build up. Monitoring compliance via on site snap shots is supported by some accreditors by required reporting and submissions but is this enough to ensuring continual assurance of patient safety? The research shows several difficulties in this approach not least of which is the lack of sustained improvement in patient safety. This presentation identifies that the cost of the current models are often unsustainable due to the significant costs experienced by both the accreditor and as a result, the accredited organization because of the need for so much onsite surveys. Change the concept of accreditation, from snap shot review, to continuous access monitoring by the accreditors and the model opens up huge potential to have a more effective and efficient, impact on patient safety. This paper presents a proposal to make this a possibility by allowing the accrediting body access to the organizations own internal systems to externally view big data in real time. Access provides the accreditor with a window into the soul of the organization, allowing for continual monitoring as deemed required. Continuous organizational access by accrediting bodies and similar external regulators is the next major step forward for patient safety evaluation. It is recognized that to achieve this, a change is required in the relationship between the accreditor and the accredited�with organizations required to show an open hand with evaluators working towards a more supportive role to assist organizations to get it right in real time. Recent Publications 1.Sweeney J, Brooks AM, Leahy A (2003) Development of the Irish National Patient Perception of Quality of Care Survey. International Journal for Quality in Health Care; 15(2): 163-168. 2.Sweeney J, Heaton C (2000) Interpretations and variations of ISO 9000 in acute health care. International Organization for Standardization. Int J Qual Health Care; 12(3): 203-9. References 1.Walshe K and Boyd A (2007) Designing regulation: A review. A review for the Healthcare Commission of the systems for regulation and their impact on the performance in seven sectors and a synthesis of the lessons and implications for regulatory design in healthcare, Manchester: Centre for Public Policy and Management. 2.Braithwaite J, Greenfield D, Westbrook J, Pawsey M et al (2010) Health service accreditation as a predictor of clinical and organizational performance: a blinded, random, stratified study. Quality and Safety in Health Care; 19: 14-21. 3.Hafner J, et al. (2011) The perceived impact of public reporting hospital performance data: interviews with hospital staff. International Journal for Quality in Health Care; 23(6): 697-704.Biography
Sweeney John Sweeney Y is the Director of Research and Development with the Irish Health Services Accreditation Board. He is Lecturer of the Royal College of Surgeons in Ireland. He has acted as the Technical Advisor to the International Society for Quality in Healthcare (ISQua) on external evaluation. In 2012, he was appointed as an International Society for Quality in Healthcare (ISQua) Expert and in 2013 he was elected to the Board of ISQua.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi