Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
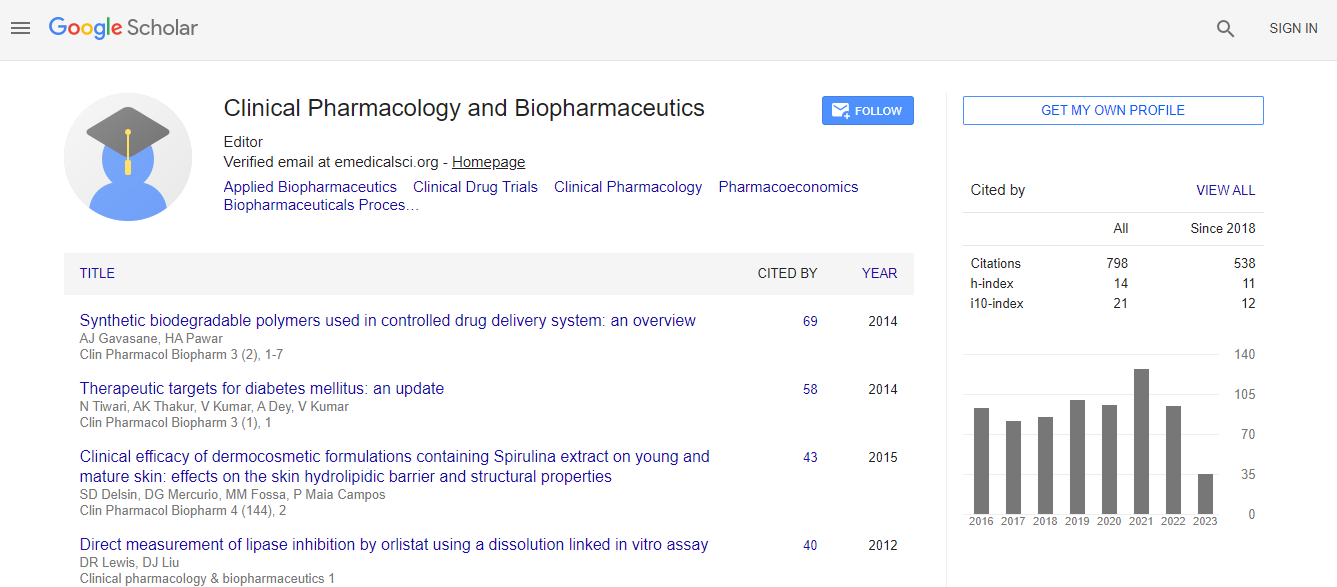
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Orthosteric- versus allosteric-dependent activation of the GABAA receptor requires numerically distinct subunit level rearrangements
Joint Event on 24th World Congress on Pharmacology & 7th World Heart Congress
Jahanshah Amin and Meena S. Subbarayan
University of South Florida, USA
ScientificTracks Abstracts: Clin Pharmacol Biopharm
Abstract
Anesthetic molecules act on synaptic transmission via the allosteric modulation of ligand-gated chloride channels, such as hetero-oligomeric α1β2γ2 GABAA receptors. To elucidate the overall activation paradigm via allosteric versus orthosteric sites, we used highly homologous, but homooligomeric, ρ1 receptors that are contrastingly insensitive to anesthetics and respond partially to several full GABA α1β2γ2 receptor agonists. Here, we co-expressed varying ratios of RNAs encoding the wild-type and the mutated ρ1 subunits, which are anesthetic-sensitive and respond with full efficacy to partial GABA agonists, to generate distinct ensembles of receptors containing five, four, three, two, one, or zero mutated subunits. Using these experiments, we then demonstrate that, in the pentamer, three anesthetic-sensitive ρ1 subunits are needed to impart full efficacy to the partial GABA agonists. By contrast, five anesthetic-sensitive subunits are required for direct activation by anesthetics alone, and only one anestheticsensitive subunit is sufficient to confer the anesthetic-dependent potentiation to the GABA current. In conclusion, our data indicate that GABA and anesthetics holistically activate the GABAA ρ1 receptor through distinct subunit level rearrangements and suggest that in contrast to the global impact of GABA via orthosteric sites, the force of anesthetics through allosteric sites may not propagate to the neighboring subunits and, thus, may have only a local and limited effect on the ρ1 GABAA receptor model system.Recent Publications:
1. Walters RJ, Hadley SH, Morris KDW, and Amin J: Benzodiazepines act upon GABAA receptors via two distinct and separable mechanisms. (2000) Nature Neuroscience; 3(12): 1274-1281.
2. W, Hadley SH, Lüddens H, Amin J: Ketamine, But Not Phencyclidine, Selectively Modulates Cerebellar GABAA Receptors Containing α6 and δ Subunits. (2008) Journal of Neuroscience 28(20): 5383-5393.
3. Morris KW and Amin J: Insight into the mechanism of action of neuroactive steroids. (2004) Mol Pharmacol; 66:56-69.
4. Hadley SH & Amin J: Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. (2007) Journal of Physiology 581.3:1001-1018.
5. Amin J, Subbarayan MS. Orthosteric-versus allosteric-dependent activation of the GABAA receptor requires numerically distinct subunit level rearrangements (2017). Scientific Reports 7 (1), 7770, 1-16.
Biography
J Amin laboratory has a primary interest in GABAA and NMDA receptor-channels. We have studied the structure/function relationship of subtypes of GABAA receptors to enhance our understanding of the molecular mechanism of action of sedative/hypnotic drugs. By co-expression of wild-type with anestheticsensitive subunits of GABAA receptors, we have determined the minimal number of subunits required for orthosteric- versus allosteric-dependent activation of GABAA receptor channels. The laboratory is also focused on drug discovery with particular interest in ketamine. In the last several years, we have synthesized a number of ketamine analogues and characterized their molecular actions on the NMDA and GABAA receptors. One oxime analogues of ketamine has shown great promise in terms of molecular signature on NMDA and GABAA receptors and in an animal model test for antidepressants.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi