Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
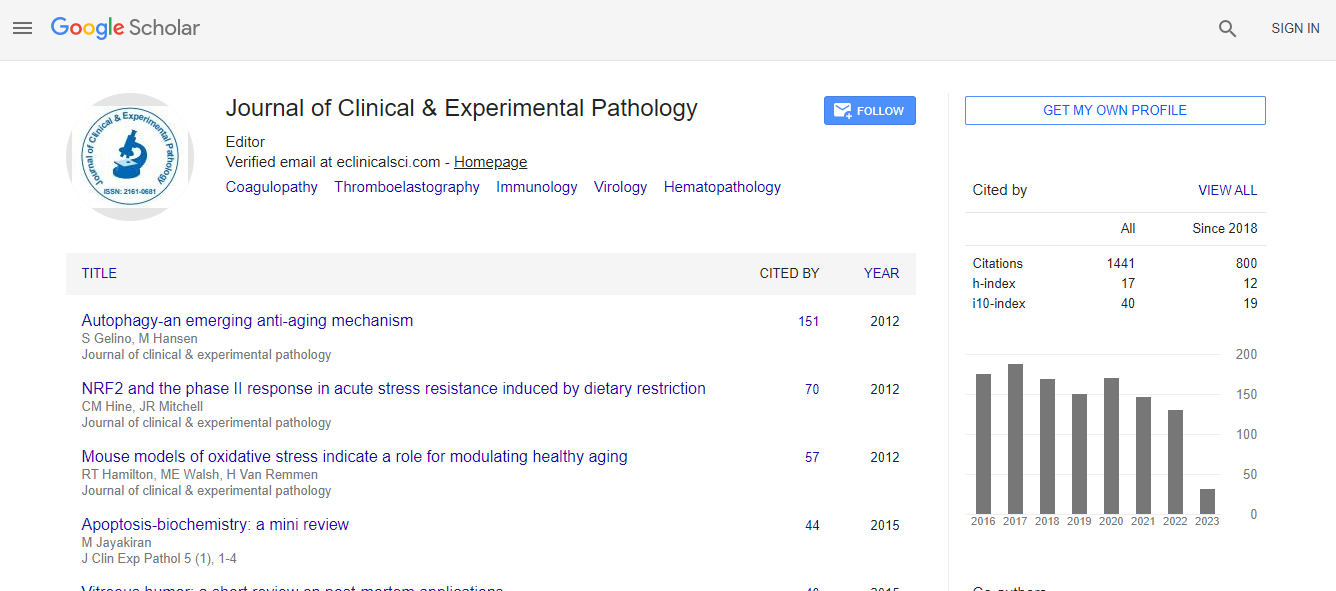
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
One-pot synthesis of oxindoles through C-H activation and evaluation of anticancer activity
Joint Event on 6th World Congress and Expo on Breast Pathology and Cancer Diagnosis & 20th International Conference on Medicinal Chemistry and Rational Drugs
Sang Hoon Han
Sungkyunkwan University, Republic of Korea
ScientificTracks Abstracts: J Clin Exp Pathol
Abstract
The oxindole skeleton has been recognized as a ubiquitous heterocycle found in bioactive natural products and synthetic compounds with medicinal applications. In particular, 3-substituted and spiro oxindole derivatives have been implicated in a wide spectrum of biological activities including serotonergic, anti-tumor, anti-Alzheimerâ�?�?s, anti-Parkinson disease, glycoprotein-mediated MDR inhibition, anti-bacterial and anti-inflammatory activities. Additionally, oxindoles serve as synthetic precursors to a range of other heterocyclic compounds including indoles and isatins. Therefore, the development of novel and highly efficient strategies for the formation of oxindole architectures is an area of great interest in organic synthesis. With recent advances in direct and catalytic Câ�?�?H functionalization, a great deal of effort has been devoted to the formation of oxindoles via transition-metal-catalyzed or metal-free oxidative Câ�?�?H functionalization events. Among reported examples, the tandem cyclization of acrylamides has attracted much attention for the synthesis of various functionalized oxindoles. Other routes rely on the Ir- or Cu-catalyzed intramolecular cyclization of �?²-keto amide derivatives. Moreover, the Ag- or Rh-catalyzed aromatic Câ�?�?H functionalization of �?±-diazoamides is another effective way to construct C3-functionalized oxindoles. However, these methods require specifically functionalized starting materials and result in a special subclass of oxindoles. With a rational design based on Câ�?�?H addition and subsequent cyclization process, we herein reported efficient access to the formation of oxindoles through Rh(III)-catalyzed site-selective alkylation of azobenzenes and internal olefins, such as maleimides, maleates and fumarates, followed by reductive intramolecular cyclization. Particularly noteworthy was the resulting 1-amino-indolic framework, which represents a biologically important scaffold found in various synthetic molecules. Thus, synthesized oxindoles were evaluated for cytotoxicity against human prostate adenocarcinoma cell lines (LNCaP), human breast cancer cell lines (MCF-7), human Ovarian Cancer Cell lines (SKOV3), human lung carcinoma cell lines (A459) and human renal adenocarcinoma cell lines (786-O).Biography
Sang Hoon Han is a student for Master and Ph.D combined Course in School of Pharmacy, SKKU.
E-mail: Sang0306@nate.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi