Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
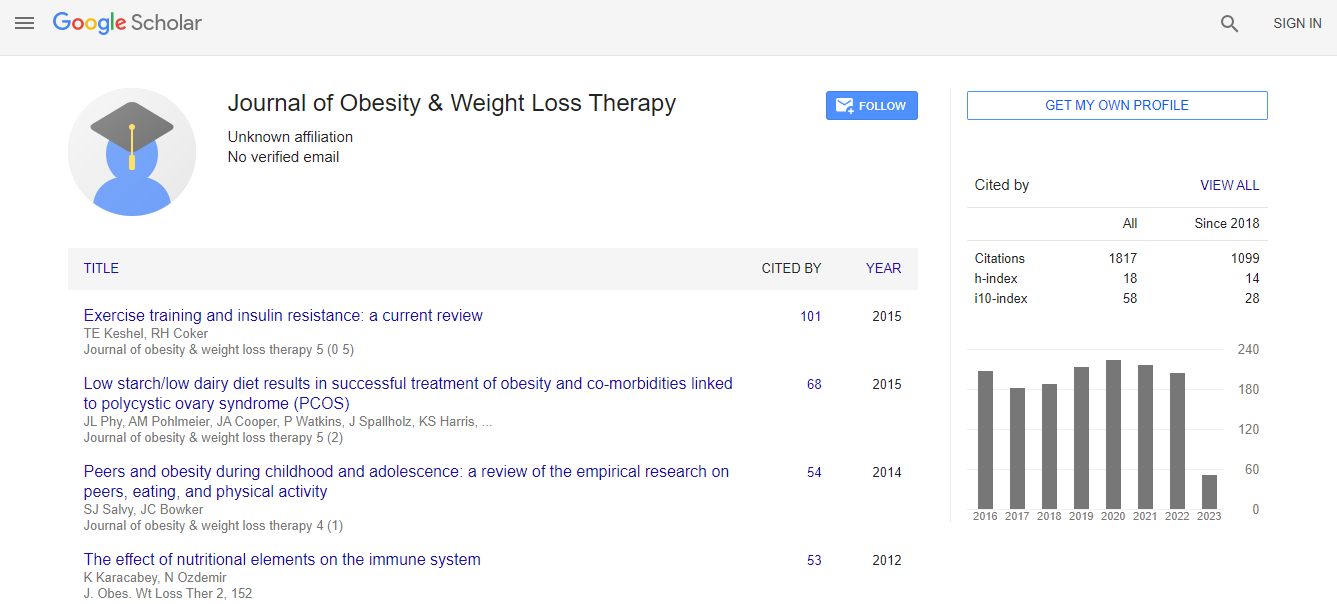
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Obesity and immune cells
7th Obesity & Endocrinology Specialists Congress
Maria Fernanda Cury-Boaventura
University of Cruzeiro do Sul, Brazil
Keynote: J Obes Weight Loss Ther
Abstract
Obesity is a major medical problem and causes the development of chronic low-grade metabolic inflammation. The high incidence of metabolic disorders is associated with white adipose tissue (WAT) surrounding intra-abdominal organs. It is believed that the initial cause of metabolic inflammation is adipocyte hypertrophy in visceral adipose tissue (VAT). In the hypertrophic adiposity, non-esterified fatty acids (NEFA) induces local macrophages to produce high levels of TNFa which in turn promote a positive feedback inducing more NEFA, pro-inflammatory cytokines, acute phase proteins and chemokines, which attract more monocytes/macrophages resulting in dysregulation of pro-inflammatory mediators andlow-grade inflammation. Hypertrophic adipose expansion also induces hypoxia that promotes angiogenic factors, hypoxia- inducible factor (HIF) 1 and inflammatory response-associated genes upregulation resulting in fibrosis. Lean adipose tissues have various anti-inflammatory immune cells, such as eosinophils, M2 macrophages, Th2 cells, iNKT cells, and Treg cells. In obese adipose tissue, the numbers of pro-inflammatory immune cells, including neutrophils, M1 macrophages, mast cells, Th1 cells, and CD8 T cells, are elevated. Simultaneously, reduced number of anti-inflammatory immune cells accelerates pro-inflammatory response and adipose tissue dysfunction. During the last decade, it was also identified that the vast majority of obese are characterized with a gut microbiota dysbiosis. Metabolic diseases are associated with cellular changes in the innate immune compartment of the intestine. The first line of intestinal defense is based on the secretion of defensins and IgA by intestinal epithelial cells, which are reduced in obese patients. The high fat diet induces the translocation of bacterial components such as LPS. The translocation of LPS or bacteria to tissues is a physiological mechanism, however, when unregulated, leads to a state of chronic inflammation that depends from the immune and epithelial cells response. A subpopulation of dendritic cells expressing CX3CR1 and innate lymphoid cells 3 are involved in the impairment of appropriate response. This mechanism is linked to the production of large numbers of cytokines such as IL-6, IL-17, IL-22, GM-CSF and TNF.Biography
Maria Fernanda Cury-Boaventura has completed her PhD in Human Physiology from University of São Paulo and Post-doctoral studies from University of São Paulo. She is a Professor and Researcher at Institute of Physical Activity and Sport Sciences since 2007. She has published more than 40 papers in reputed journals and has been serving as an Editorial Board Member of repute.
Email: mafecuryb2009@hotmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi