Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
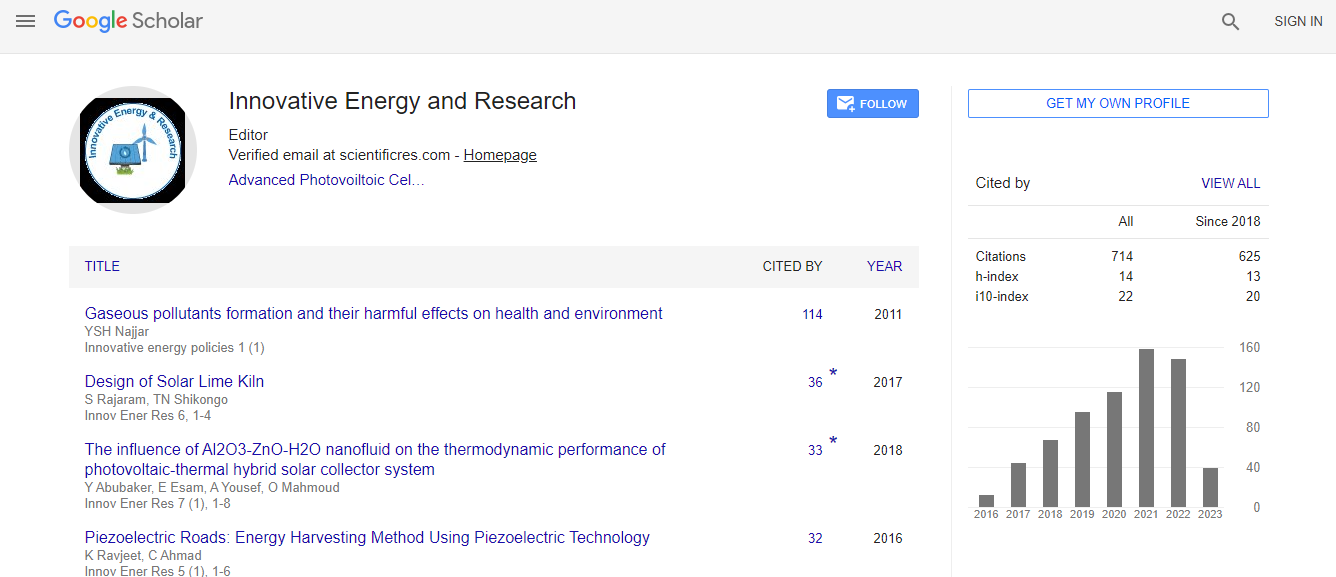
Google Scholar citation report
Citations : 712
Innovative Energy & Research received 712 citations as per Google Scholar report
Innovative Energy & Research peer review process verified at publons
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Novel polyolefin-based proton exchange membranes for fuel cell applications
21st International Conference on Advanced Energy Materials and Research
T C Mike Chung
The Pennsylvania State University, USA
Posters & Accepted Abstracts: Innov Ener Res
Abstract
A new class of polyolefin based proton exchange membranes are formed by a well-defined graft copolymer that contains a high molecular weight semicrystalline PE or PVDF backbone and several amorphous sulfonated poly(arylene ether sulfone) (s-PAES) or sulfonated polystyrene (s-PS) side chains. The suitable chemical routes will be discussed for preparing the graft copolymers with desirable backbone molecular weight, graft density, graft length, sulfonic acid concentration, etc. One example is PE-g-s-PAES copolymer that is prepared by a graft onto reaction between high molecular weight HDPE with few benzyl bromide groups and poly(arylene ether sulfone) (PAES) with two terminal phenol groups. The resulting PE-g-PAES graft copolymer was solution cast into uniform film (thickness 20–40 μm), followed by a heterogeneous sulfonation reaction to obtain PE-g-s-PAES PEMs. The unique combination of hydrophobicity, semicrystallinity and high molecular weight of PE backbone offers PEM with a stable (non swellable) matrix. On the other hand, the hydrophilic proton conductive s-PAES side chains in the PE-g-s-PAES membrane self-assemble into many conducting channels. In addition, a thin PE layer spontaneously formed on the membrane surfaces. In the bulk, these membranes show good mechanical properties (tensile strength >30 MPa, Young’s modulus >1400 MPa) and low water swelling (λ <15) even with high IEC >3 mmol/g in the s-PAES domains. Compared to Nafion 117, most PE-g-s-PAES PEMs show similar hydration numbers (λ <15) but higher proton conductivity (up to 160 mS/cm). More interestingly, all PE-g-s-PAES PEMs show higher through-plane conductivity than in-plane conductivity. In direct methanol fuel cells, the PE surface layer also minimizes methanol cross over from anode to cathode with reduced fuel loss, and stops the HO• and HO2• radicals, originally formed at the anode, entering PEM matrix. Overall, this newly developed PE-g-s-PAES membrane offers a combination of desirable properties, including conductivity, water uptake, selectivity, mechanical strength, and cost-effectiveness for fuel cell applications.Biography
E-mail: chung@psu.edu

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi