Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
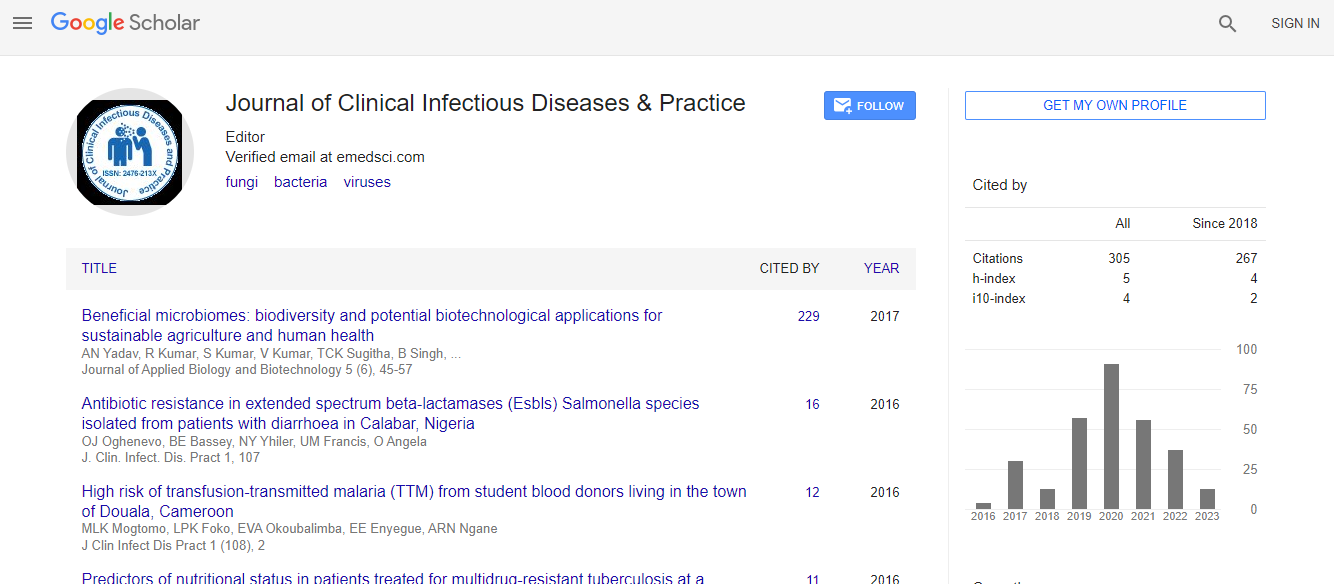
Google Scholar citation report
Citations : 531
Journal of Clinical Infectious Diseases & Practice peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
Nitisinone in the treatment of alkaptonuria
9th World Congress on Rare Diseases and Orphan Drugs
Lakshminarayan Ranganath
Royal Liverpool University Hospital, UK
ScientificTracks Abstracts: J Clin Infect Dis Pract
Abstract
Alkaptonuria (AKU) is an iconic autosomal recessive severe multisystem disorder of the tyrosine degradation pathway due to lack of homogentisate dioxygenase resulting in increased circulating and urinary homogentisic acid. Morbidity includes lithiasis (renal, salivary, prostate, gall bladder), osteopenia, fractures, ruptures of ligaments/ muscle/tendons, spine and joint disease. An approach to treating AKU by inhibiting the production of HGA by using nitisinone has been recently recognized. Nitisinone has been used in a related tyrosine disorder, hereditary tyrosinaemia 1 (fatal in early childhood) as the standard of care for more than 20 years. This presentation discusses the efforts of our group in developing nitisinone for AKU, an approach consistent with repurposing. Nitisinone is being developed as a licensed therapy in DevelopAKUre, a European Union funded clinical programme. In parallel, nitisinone is also being used off license in a centre (NAC) commissioned by NHS England Highly Specialised Services since 2012. Data collected from the NAC shows a beneficial effect of nitisinone in AKU.Biography
Lakshminarayan Ranganath is a Consultant in Clinical Biochemistry and Metabolic Medicine at the Royal Liverpool Hospital. He has completed his Graduation in Medicine from Madras before moving to UK. He has completed a Clinical and Research Training in Surrey before moving to Liverpool in 1999 where he presently works. He has established the AKU theme of clinical and basic science research in 2003. He is now a leader in this field. He has published over 100 papers. He is the Chief Investigator and Coordinating Investigator of SONIA clinical trials. He is the inaugural Clinical Director of the Robert Gregory National AKU Centre in Liverpool.
E-mail: lrang@liverpool.ac.uk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi