Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
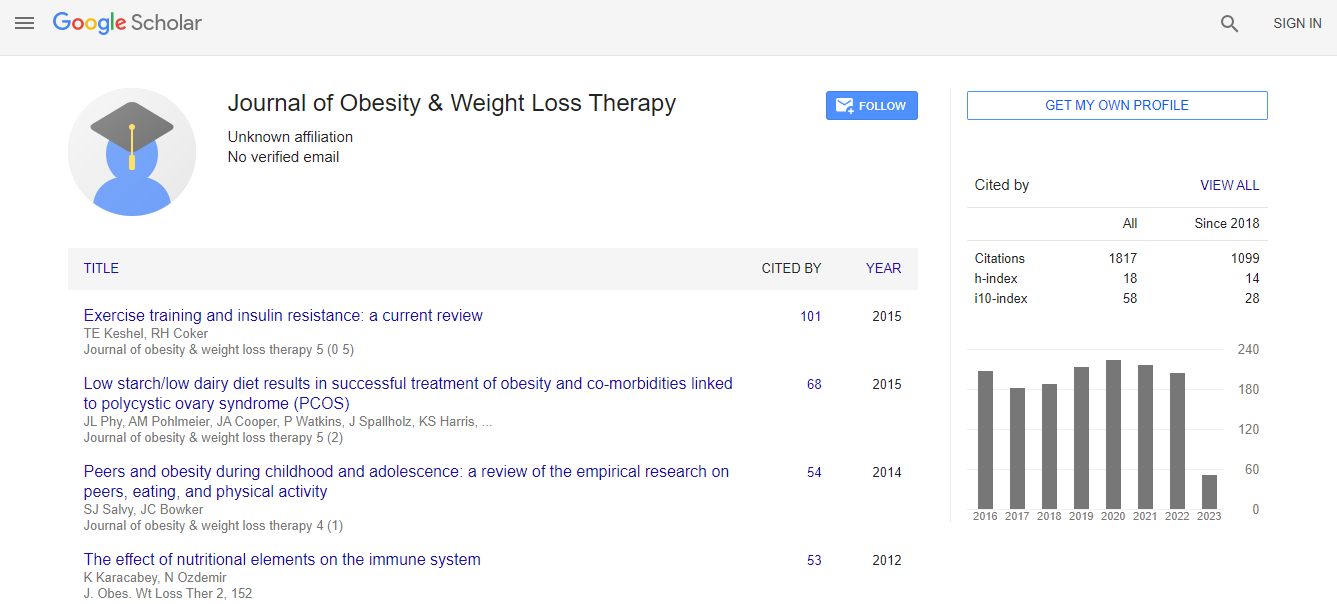
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
New approaches towards the pharmacological treatment of obesity
5th International Conference on Obesity and Diet Imbalance
Dr Marilena Vlachou
Department of Pharmaceutical Technology, School of Pharmacy, National and Kapodistrian University of Athens, Greece
ScientificTracks Abstracts: J Obes Weight Loss Ther
Abstract
Obesity is a condition defined as excess body fat due to positive energy balance. It is often associated with metabolic diseases, such as hypertension, type 2 diabetes, and coronary heart disease. Obesity also increases the risk of several malignancies in colon, breast, pancreas, and endometrium. Treatments of obesity include lifestyle interventions (dietary interventions with hypocaloric diets that are low in fats and carbohydrates and/or exercise), pharmacotherapy, and surgery. If weight loss with lifestyle changes is only modest, pharmacotherapy might be needed. Pharmacotherapy, always adjunctively with lifestyle interventions, is also an option for any patient diagnosed with obesity (body mass index [BMI] of 30 kg/m2 or greater) or with a BMI of 27 kg/m2 or greater and at least one coexisting condition, including type 2 diabetes, hypertension, hyperlipidemia, and sleep apnea. Presently, six drugs, including two peptides (liraglutide and semaglutide) are approved for weight management in adults: orlistat, phentermine, phenterminetopiramate and naltrexone-bupropion. Orlistat is approved in both Europe and USA and its mode of action is by energy waste. Phentermine, phenterminetopiramate and lorcaserin are approved only in the US and their action is related to appetite reduction. The same mode of action is followed by naltrexone-bupropion and liraglutide, which are approved in both Europe and USA. From the aforementioned drugs some are approved for short-term management (< 6 months) whilst others are approved for long-term management (> 12 months). The drug therapy is personalized and modified for each individual patient, depending on needs, contraindications, and cost. Benefits of these drugs should be assessed regularly (every 2-3 months) and a different drug treatment should be considered if at least 5% of body weight is not lost after 3 months of therapy. As already mentioned, one of the approved drugs in obesity management is the combination of naltrexone/bupropion, which was developed by joining these two brain regions acting agents, already approved for other indications (naltrexone for opioid and alcohol addiction and bupropion for depression and smoking cessation), in a single solid pharmaceutical formulation, which regulates food intake and body weight. This compounded drug is known as Contrave├?┬« in the US or Mysimba├?┬« in Europe (Orexigen Therapeutics, Inc.). However, there are concerns regarding cardiovascular-related side effects of these naltrexone/bupropion carriers. Due to these serious health adverse effects, there is an urgent need to develop more effective and less hazardous naltrexone/bupropion sustained/controlled release systems, by employing biopolymeric excipients with stereoelectronic characteristics that would favor chemical interactions with the pharmacophoric groups of both naltrexone and bupropion. These inter- and supra-molecular interactions will allow for maximum therapeutic potential and substantially less side effects. To conclude, obesity is a multifactorial disease, which poses serious health risks. Its timely and correct treatment is a panacea for the course of mental and physical health. A cornerstone for the treatment of obesity is the healthy dietary/exercise interventions and possible the inclusion of preparations with satisfactory effectiveness and safety. Still, research and development should continue the effort to develop new agents and drug combinations. Importance of Research: As previously stated, obesity, which affects about 13% of the world population, results in significant deterioration of health and serious clinical, mainly metabolic and cardiovascular complications. Regarding the current alarming epidemiological data there is a need for intensive prevention and treatment of obesity and the development of new forms of pharmacotherapy (new treatment regimens) to develop effective, safe, long-term effective therapy for the treatment of obesity, and above all, to individualize therapy. However, the formulation development and the excipient selection are time consuming processes that delay the release of the drug to the market. Our research team is currently working on the production of multilayered tablets of naltrexone/bupropion, which can offer anti-obesity treatment. This is envisioned to take place by using, for the preparation of the proposed tablets, combinations of biopolymers with physicochemical properties compatible with the stereoelectronic features of both naltrexone and bupropion. This will lead to the release of the compounded two drugs by an overlapping sustained/controlled mechanism. This combined release mechanistic model, which will be used for the first time, in the case of the naltrexone/bupropion system, is expected to result to effective antiobesity therapy with less adverse effects.Biography
Marilena Vlachou is an Associate Professor at the National and Kapodistrian University of Athens (NKUoA), Greece. She obtained her Pharmacy and PhD (Pharmaceutical Technology) degrees from the NKUoA. Just prior to obtaining her PhD degree she moved to the University of Rhode Island, U.S.A., as a Visiting Research Scientist, to conduct state of the art research related to Pharmaceutical Technology techniques. In NKUoA, she teaches, at both undergraduate and postgraduate level, courses related to the fields of Pharmaceutical Technology, Physical Pharmacy and Nanotechnology. She has co-authored the textbook entitled “Pharmaceutical Technology I: Principles of Physical Pharmacy and Nanotechnology”, and many book chapters. She has presented her research work in more than sixty (60) International Scientific Conferences and she has published more than fifty (50) articles in peer-reviewed Journals.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi