Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
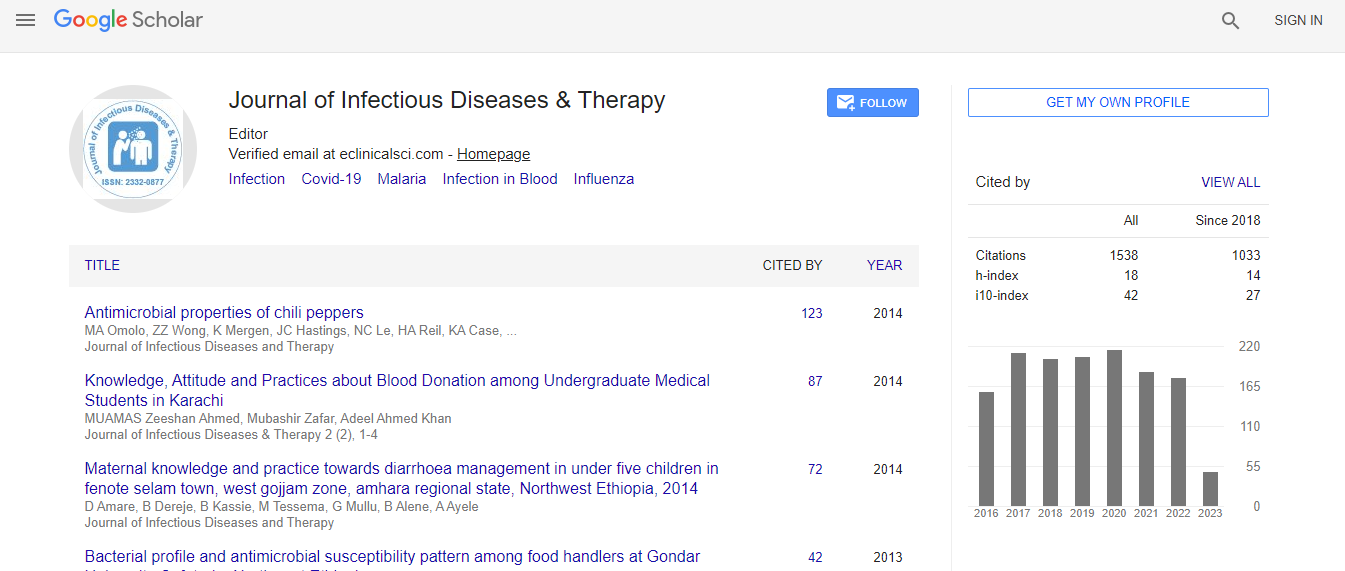
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Medical device reprocessing (MDR) in Alberta medical clinics: Patient safety risk warrants regulatory oversight
4th World Congress on Infection Prevention and Control
Benjamin Kung
College of Physicians and Surgeons of Alberta, Canada
Posters & Accepted Abstracts: J Infect Dis Ther
Abstract
Historically in Alberta, responsibility for monitoring infection prevention & control (IPAC) in non-governmental, unaccredited medical clinics had fallen on the business owner and/or physicians. In 2007, a sentinel event triggered a government directed review of IPAC in these settings. A program was created under the direction of a 10-member Advisory Committee: Infectious disease specialists, medical officers of health, senior infection control practitioners, and community physicians and surgeons. CPSA has since actively monitored IPAC with a priority on standards for MDR (cleaning, disinfection and sterilization of medical devices). Alberta has approximately 1700 medical facilities in the �non-governmental, unaccredited�category. Over 600 (>35%) perform some type of MDR and these were assessed for adherence to standards during 2008-2015. In 2013, a provincial policy for reporting the most critical deficiencies was formalized. From 2013-15, 131 assessments identified 17 (13.0%) with risks exceeding the reporting threshold to public health. Deficiencies contributing to the likelihood of reporting included but were not limited to inadequate device cleaning, lack of monitoring sterilization cycles for physical (time, temperature), chemical and/or biological parameters, use of unlicensed sterilizers and inadequate level of reprocessing given device risk classification (Spaulding�s). Post-exposure risk assessment deemed four (3.0%, n=131) a sufficient threat to initiate look backs for blood-borne pathogen exposure (HIV, HBV & HCV). Formal reporting and post-exposure risk assessment confirmed initial observations suggesting clinics performing MDR are at elevated risk of breaching IPAC principles that may jeopardize patient safety. The logistics and value of providing clinic support via robust regulatory controls is worth exploring.Biography
Benjamin Kung is the Program Manager of IPAC for the CPSA and additionally serves as Governance Committee Chair on the Board of Directors, Alberta Public Health Association. He has completed his undergraduate training in Microbiology/Biology from University of Victoria and Environmental Public Health from British Columbia Institute of Technology, followed by graduate studies in IPAC from University of British Columbia and Epidemiology at London School of Hygiene & Tropical Medicine.
Email: benjamin.kung@cpsa.ab.ca

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi