Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
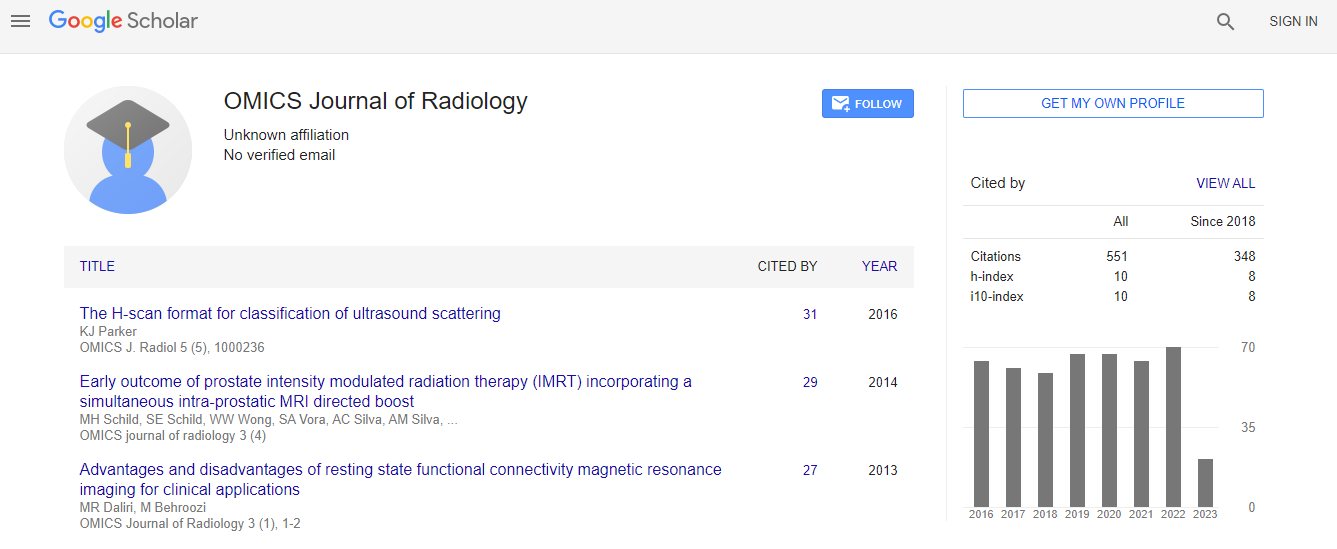
Google Scholar citation report
Citations : 551
Journal of Radiology received 551 citations as per Google Scholar report
Journal of Radiology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Share This Page
Judging response to cancer therapy RECIST and Beyond
2nd World Congress on Radiology and Oncology
Arvind K Chaturvedi
Rajiv Gandhi Cancer Institute & Research Centre, India
Keynote: OMICS J Radiol
Abstract
Monitoring response after treatment of cancer is an integral component of oncology practice. Objective tumor shrinkage has been widely regarded as a standard to judge response and is routinely used in everyday clinical practice to guide clinical decision-making. Imaging studies play a critical role in quantifying tumor response. The World Health Organization in 1979 laid down the WHO criteria for response assessment. The European organization for research and treatment of cancer came up with Response Evaluation Criteria in Solid Tumors in the year 2000 (RECIST). The RECIST documentation goes beyond lesion selection, measurement and assessment of response. It also makes specific recommendations on the usage of imaging techniques. RECIST was modified in 2009 to RECIST 1.1 which is the current standard for objective response assessment in most solid tumors. However, both WHO and RECIST criteria have relied upon size alone. It is well-known that cancer response to treatment is not always by reduction in size alone. RECIST doesn't work very well with Gastro Intestinal Stromal Tumors (GIST), mesotheliomas and Hepato Cellular Carcinoma (HCC) after locoregional therapies such as TACE and ablative treatments. For this reason, modified RECIST criteria (mRECIST) for HCC and Choi criteria for GIST have evolved. With many new anti-cancer drugs, particularly molecular targeted therapies, decrease in metabolic activity precedes any reduction in size. Also, very often as in lymphomas a non-viable residual mass without any viable tumor tissue may continue to be seen. As such PET-CT is being increasingly used today to monitor response. It is a part of the new PERCIST criteria and is the standard tool in assessing response in lymphomas. With increasing use of molecular targeted therapies and immunotherapy to treat many advanced cancers there is a fundamental change in the way cancers may respond. Cancer specific and therapy specific response criteria have become relevant in an era of personalized medicine. Paradoxically increase in size and even appearance of a new lesion may well be a part of the initial response in immunotherapy. The evolution of response criteria, going beyond RECIST and evaluation of cancer and therapy specific response is the primary objective of this study.Biography
Arvind K Chaturvedi is currently the Chair of the Department of Radiology at the Rajiv Gandhi Cancer Institute & Research Centre, India. He has also served as the Medical Director of the Institute from 2006 to 2010. He is directing Oncological Radiology Fellowship program and has the distinction of having trained many international radiologists. He is a Member of Radiological Society of North America, European Society of Radiology, Breast Imaging Society of India and Indian Radiological and Imaging Association.
E-mail: arvindatdelhi@yahoo.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi