Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
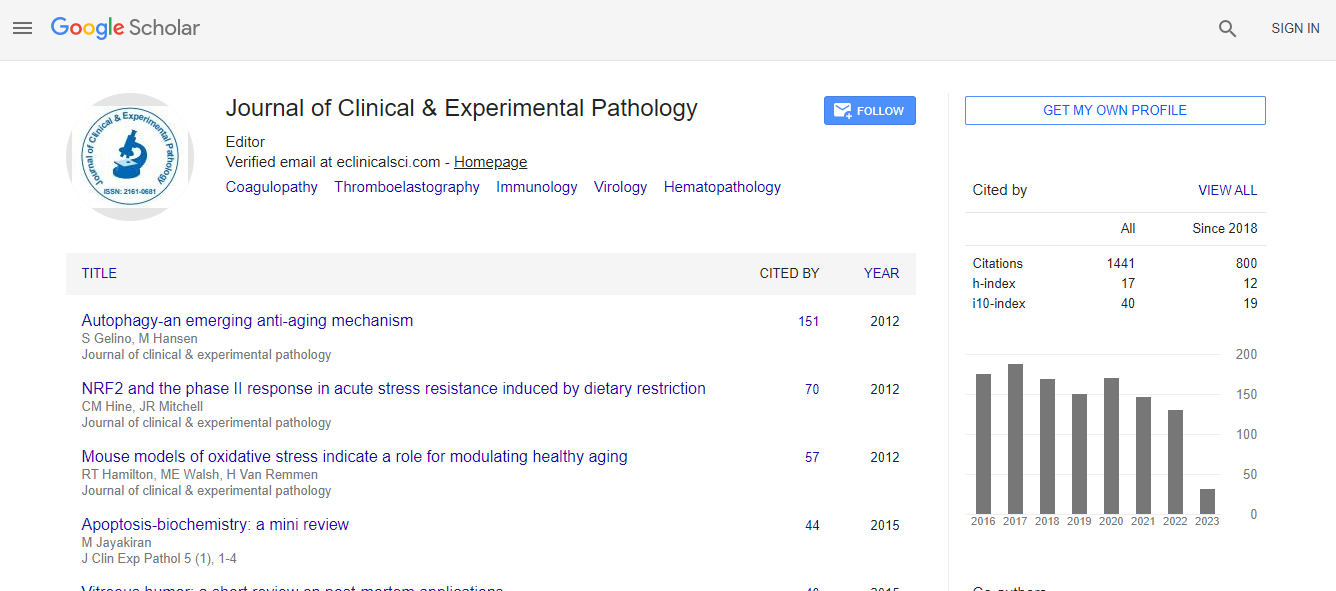
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Integrating next generation sequencing in to clinical laboratories for cancer patient management
7th World Congress on Molecular Pathology
Rajyalakshmi Luthra
The University of Texas, USA
Posters & Accepted Abstracts: J Clin Exp Pathol
Abstract
Cancer is a heterogeneous and highly complex disease of the genome. Sequencing cancer genome to detect genetic aberrations is the hallmark of precision cancer medicine. In the past, Sanger sequencing and pyrosequencing, the gold standard conventional sequencing techniques were used extensively for cancer genome analysis. These low and medium-throughput sequencing technologies dominated the clinical arena until the recent development of next generation sequencing technologies (NGS) that have revolutionized the field of clinical cancer genomics. Massively parallel sequencing capacity of NGS facilitates simultaneous screening of large areas of the genome in multiple samples. The combination of high-throughput capability, decreased costs and clinical utility has led to widespread and rapid adoption and integration of NGS-based mutational analysis into the real-time management of patients with cancer. However, the implementation of NGS in a clinical laboratory presents challenges due to complexity associated with technology as well as the data analysis and medico-legal implications of the data generated. This presentation will discuss the advantages and challenges associated with clinical NGS implementation in routine diagnostics and the utility of unprecedented potential of NGS as the new ├ó┬?┬?gold standard├ó┬?┬? to explore cancer genome evolution and tumor heterogeneity.Biography
Email: rluthra@mdanderson.org

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi