Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
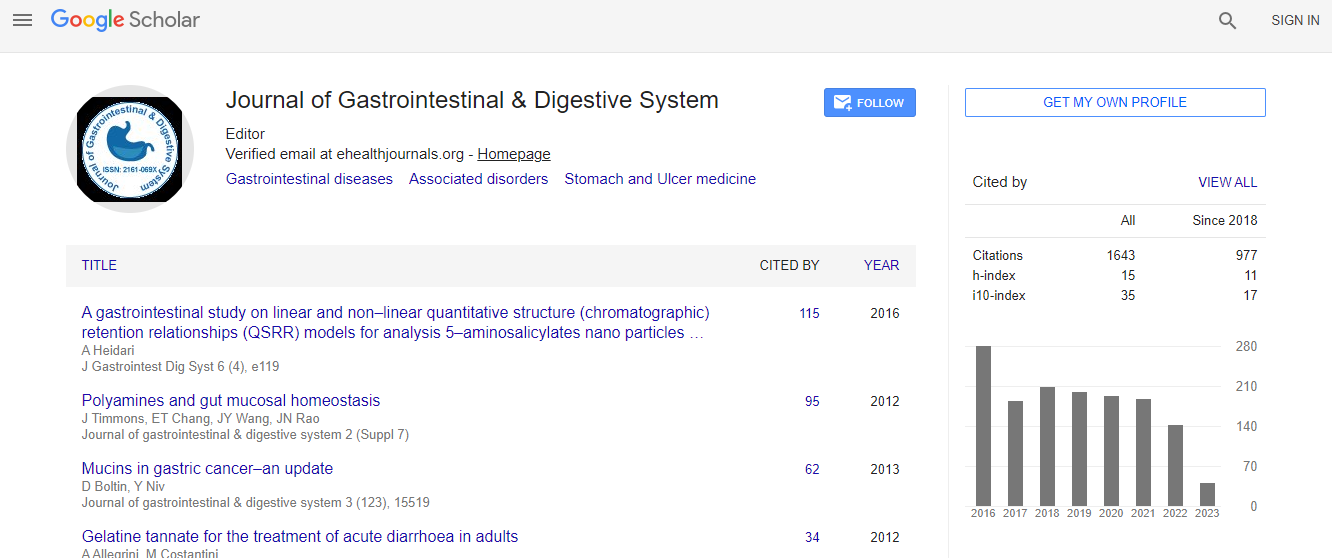
Google Scholar citation report
Citations : 2091
Journal of Gastrointestinal & Digestive System received 2091 citations as per Google Scholar report
Journal of Gastrointestinal & Digestive System peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Infliximab biosimilars (RemsimaTH) in therapy of IBD patients: Experience form one tertiary IBD center
13th International Conference on Clinical Gastroenterology, Hepatology and Endoscopy
Milan Lukas
Charles University, Czech Republic
Keynote: J Gastrointest Dig Syst
Abstract
Background: The evidence on efficacy and safety of biosimilars infliximab (IFX) in patients with inflammatory bowel diseases (IBD) is sparse. Methods: One cohort composed of prospectively followed patients who were switched from original to biosimilars IFX. The second cohort included retrospectively assessed anti-TNF├?┬?├?┬▒ na├?┬?├?┬»ve patients who started therapy. Disease activity was assessed using standard clinical indices, endoscopic evaluation, and laboratory parameters. Trough levels (TL) and anti-drug antibodies (ATI) were also measured. Patients were evaluated 56 weeks (W56) after switch and at weeks 14 (W14) and 46 (W46) in the na├?┬?├?┬»ve cohort. Results: 74 IBD patients were switched to biosimilars IFX and 119 na├?┬?├?┬»ve patients newly initiated therapy with the preparation. Disease activity remained stable in majority of switched patients (remission at W0 vs. W56: 72.2% vs. 77.8%; median difference of both HBI and SCCAI between W0 and W56 was 0). Comparing W0 and W56, no significant difference in CRP and FC was observed. In total, 92% of CD and 83% of UC patients responded to induction therapy (W14) with biosimilars IFX. At W46 the response rate was 86% in CD and 64% in UC. Moreover, half of UC patients experienced mucosal healing at W14 and improvement of perianal disease occurred in 95% of CD at W46. No increase in immunogenicity was found in switched patients and type and frequency of adverse events were comparable to original preparation. Conclusion: Infliximab CT-P13 is affordable therapy in IBD patients.Biography
Milan Lukas is a Professor in ISCARE Lighthouse Clinical Center IBD Clinical and Research Centre ISCARE and 1st Medical Faculty in Charles University, Czech Republic.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi