Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
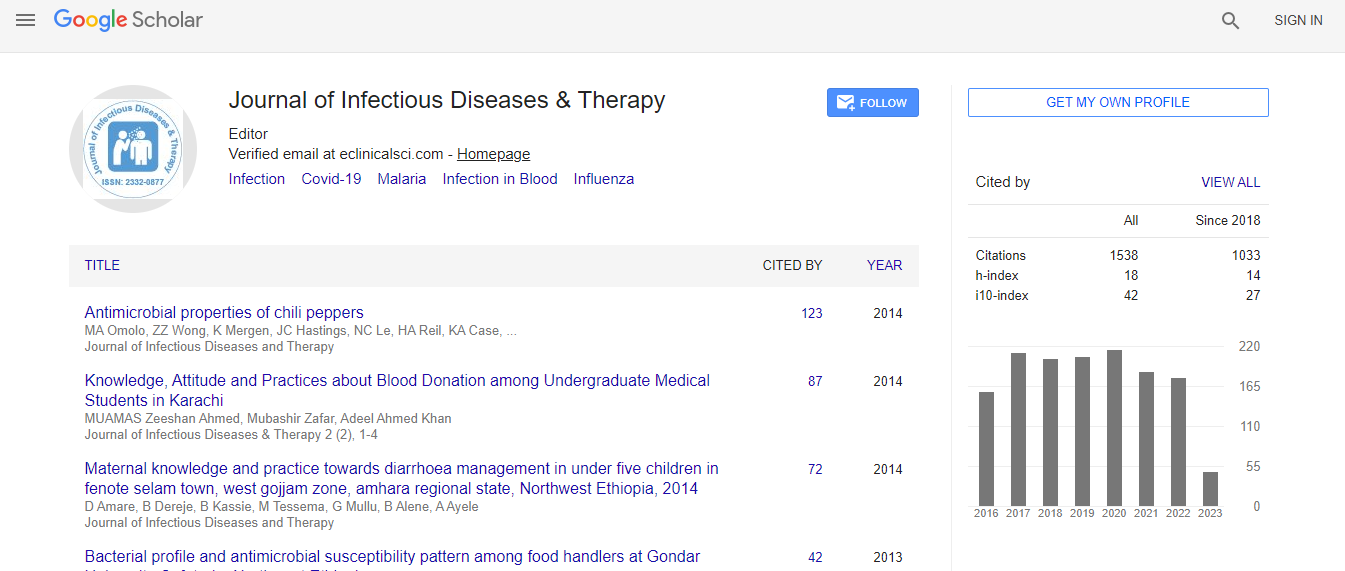
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
In vivo, in vitro interaction of silver nanoparticles with leucine amino peptidase from human and Plasmodium falciparum
3rd Annual Congress on Infectious Diseases
Chris Whiteley
Rhodes University, South Africa
ScientificTracks Abstracts: J Infect Dis Ther
Abstract
Statement of the Problem: There is increasing requirement for the development of new drug protocols against malaria, a fatal disease caused by the lethal parasite Plasmodium falciparum. Leucine aminopeptidase (PfLAP) of Plasmodium falciparum is being pursued as a promising target for the discovery of novel antimalarials. Methodology: PfLAP and HsLAP were expressed in Escherichia coli, and AgNPs (3-10 nm) characterized by ultra-violet spectroscopy and transmission electron microscopy. The effects of silver nanoparticles (AgNPs) against P. falciparum leucine amino-peptidase (PfLAP) and the human homolog (HsLAP) were compared. Findings: PfLAP indicated a Km of 694 �¼M towards leucine-p-nitroanilide and a Vmax of 57.9 �¼mol.ml-1. min-1 while HsLAP had a Km of 1.6 mM and Vmax of 119.6 �¼mol.ml-1.min-1. On interaction with AgNPs (670 nM) PfLAP was selectively inhibited (57.1 %; Ki=610 nM) relative to HsLAP (10.8 %; Ki=5.22 �¼M). The viability of P. falciparum parasites was decreased when exposed to silver nanoparticles, with an IC50 value of 6.96 �¼M, compared to an IC50 value of 647.7 �¼M for human HeLa cells. Conclusion & Significance: Structural differences between the enzyme variants, particularly the orientation and distance of surface Met349 in PfLAP and Met306 in HsLAP to the zinc binding sites were significant and may allow for selective targeting of PfLAP by AgNPs.Biography
Chris Whiteley is an Emeritus Professor of Biochemistry at Rhodes University, Grahamstown, South Africa and distinguished Research Professor at National Taiwan University Science & Technology, Visiting International Professor in Enzymology at School of Bioscience & BioEngineering of South China University Technology, Guangzhou, PRC. He served as Visiting Research Scientist at the Department of Chemical Engineering, National Taiwan University, Taipei, Taiwan in 2004 and as Visiting Professor of Biochemistry at Institute of Biomedical Technology, Veterans General Hospital, Yang Ming University, Taipei, Taiwan. He also worked as Visiting Professor of Enzymology & Organic Synthesis at Oregon State University, Corvallis, Oregon, USA and Visiting Professor of Organic Synthesis at University British Columbia, Vancouver, Canada. He is the Executive Member of Royal Chemical Society (London), MRSC (C. Chem), South African Chemical Institute (SACI). He has published 6 chapters in books and has 110 peer-reviewed papers on Biomedical Enzymology and Nanomaterials.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi