Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
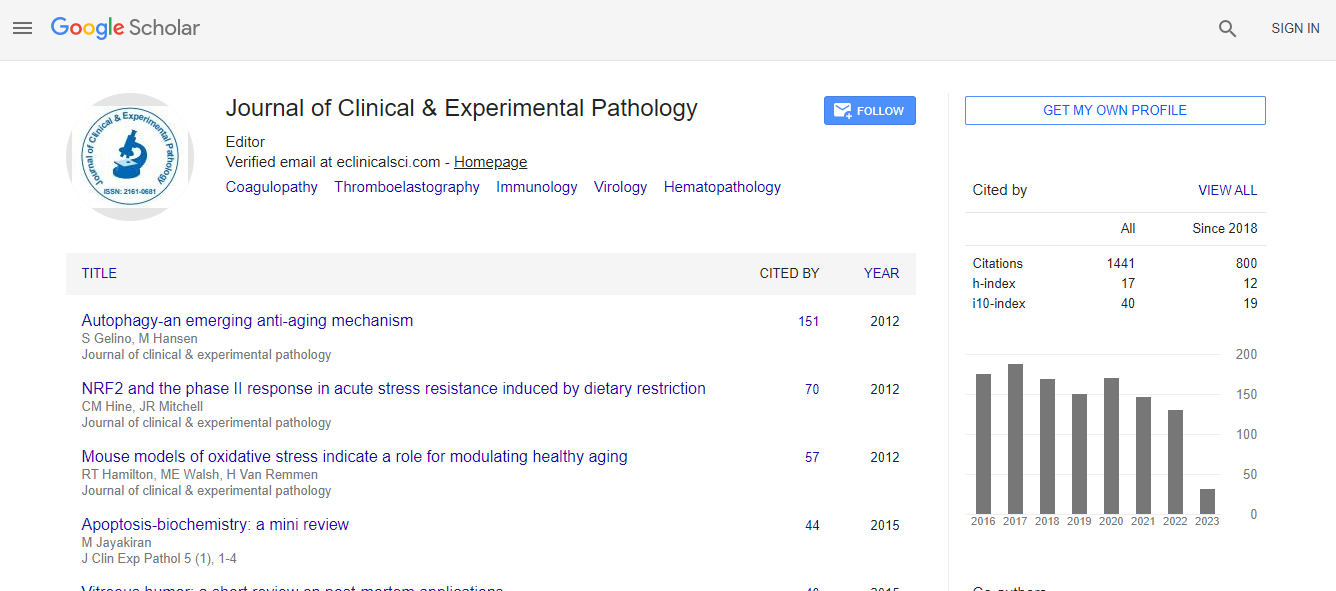
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Implementation of NGS causes dynamic shifts in clinical molecular diagnostics
12th International Conference on Pediatric Pathology & Laboratory Medicine
Petr Starostik
University of Florida, USA
Keynote: J Clin Exp Pathol
Abstract
The past decade witnessed a true revolutionary change in ways how mutation detection is performed in a clinical laboratory. Single analyte tests were replaced in many labs by multianalyte next-generation sequencing (NGS). This technique significantly decreased the sequencing cost per-base, enabled labs to analyze much higher number of samples at once, and broadened the analysis scope from a single gene to gene panels or even the whole exome/genome. Many new so far unknown gene mutations were discovered by NGS. They can be used as biomarkers in diagnosis and their availability led to changes in tumor classification. They are also potential drug targets to develop targeted therapies. Several manufactures supply NGS instruments and reagents to detect both somatic and germline mutations. Many laboratories opt to develop their own laboratory-developed NGS assays which can be easily tailored to meet their needs. We developed both amplicon- and hybridization probe-based NGS assays used to detect driver and druggable mutations in different types of cancers. The assays were extensively validated, and allow for quick and sensitive detection of point mutations and indels for the most relevant therapeutic genes in several types of cancers. The complexity of NGS does not make its implementation easy. NGS wet lab workflow entails several critical steps like sample and sequencing library preparation which are critical for success. Bioinformatics is an integral part of NGS and needs to be handled by an experienced IT specialist to not only develop appropriate analysis pipeline but to also make the results available in the appropriate format in the electronic medical records. Administrative leadership is needed to secure proper reimbursement and keep track of government regulations and oversight.Biography
Petr Starostik is an Associate Professor of Pathology and serves as the Director of Molecular Pathology in the Department of Pathology, Immunology, and Laboratory Medicine in the College of Medicine, University of Florida in Gainesville, FL. Over the years, he directed several molecular diagnostics laboratories, both in the USA and abroad. Development of Molecular Diagnostic Tests is his specialty as evidenced by his publications and the multitude of laboratory-developed tests performed in laboratories he directed. Besides clinical work, he also pursues basic research focusing on the role of FLT3 ITD in acute leukemia.
Email: starostik@pathology.ufl.edu

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi