Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
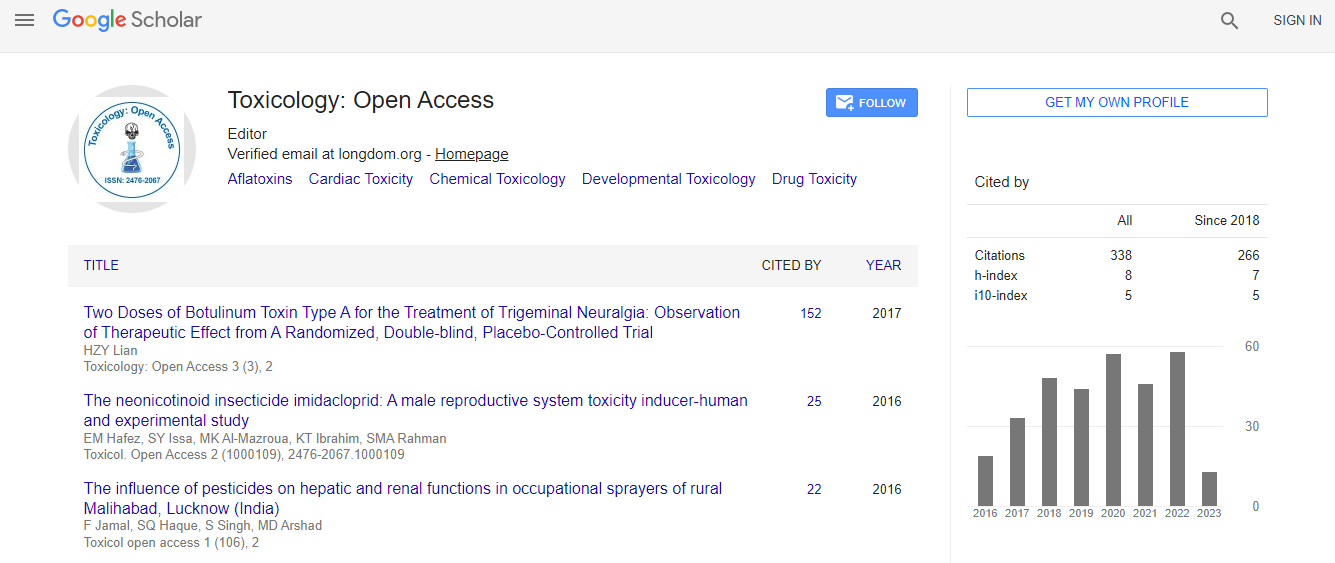
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Hazard identification in newly developed antimicrobials
8th World Congress on Toxicology and Pharmacology
Kejlova Kristina, Bendova Hana, Sosnovcova Jitka, Chrz Jan and Dvoakova Marketa
National Institute of Public Health, Czech Republic
Posters & Accepted Abstracts: Toxicol Open Access
Abstract
Protection of consumer products such as food contact plastics, coatings, cosmetics and textiles against undesirable microbial attack requires innovative agents with a wide spectrum of efficiency, long term stability and safety of use. These requirements are often difficult to meet when using current biocides and antimicrobials. The aim of the currently performed research project ALTERBIO is to identify and select innovative and efficient antimicrobial agents, based on silver nanoparticles and photoactive phthalocyanine derivatives, able of covalent or ionic bond within a polymeric system and without undesirable effects on human health and the environment. Within the project, the promising agents with proved efficient and stable antimicrobial effects need to be subjected to a battery of toxicological tests to avoid local and systemic toxicity hazard. The battery of toxicological test to identify local toxicity includes namely skin and eye irritation/corrosion, phototoxicity and skin penetration. The basic systemic toxicity tests comprise acute toxicity, genotoxicity, skin sensitization and endocrine disruption. In compliance with the current European legislation restricting the use of experimental animals the toxicological methods employed in the project comprise exclusively in vitro procedures based on cellular and tissue models either of human origin or mimicking human tissues. The presented poster summarizes the available methods and obtained results.Biography
Kejlová Kristina graduated from the Charles University in Prague and received her PhD at the Palacký University in Olomouc. She works as the Head of Unit for Alternative Toxicological Methods at the National Institute of Public Health in Prague, Czech Republic. She is an OECD and EURL-ECVAM expert for in vitro methods. She has published more than 50 papers as author/co-author of reputed journals.
Email: kristina.kejlova@szu.cz

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi