Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
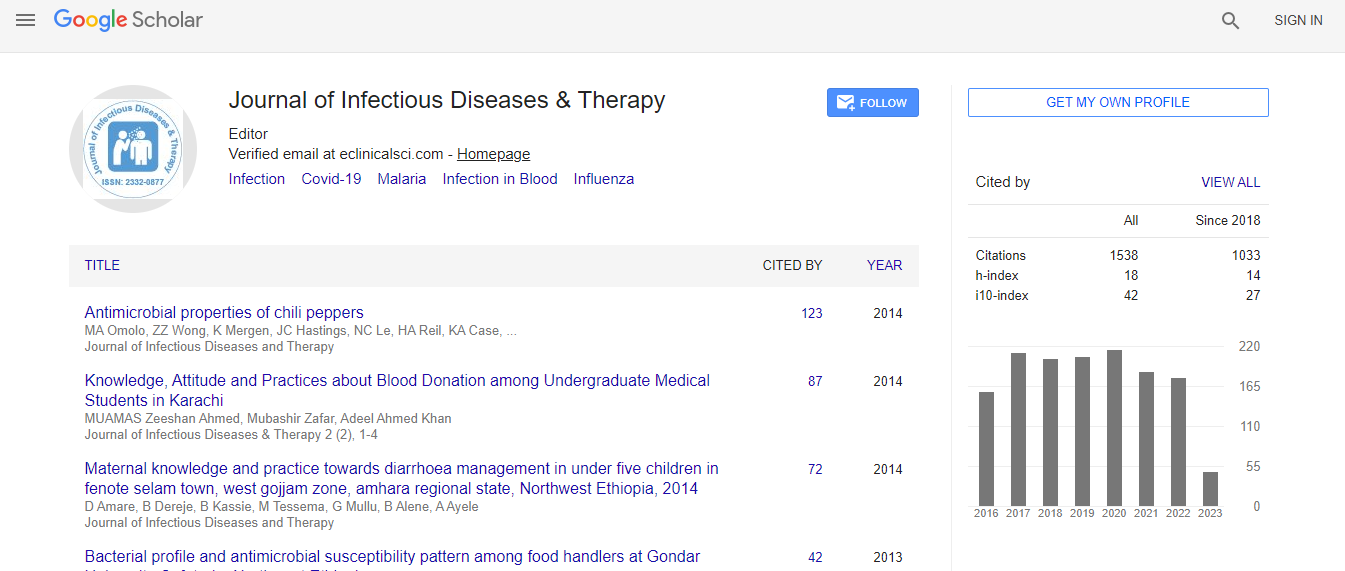
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
H7N9 influenza virus infection in BALB/c mouse induced impaired neutralizing antibody responses
International Conference on Influenza
Anna J X Zhang, Andrew C Y Lee, Houshun Zhu, Can Li, Chuangen Li, Pui Wang and Kwok-Yung Yuen
The University of Hong Kong, Hong Kong
Posters-Accepted Abstracts: J Infect Dis Ther
Abstract
Background: Novel avian H7N9 influenza virus infects humans causing severe respiratory diseases which lead to a case mortality higher than 30%. Genomic analysis showed that these H7N9 viruses contained relative low frequency of T cell epitopes on their HA protein compared to other subtypes of influenza viruses, suggesting that these viruses have low immunogenicity. In this study, serum antibody responses to H7N9 infection in BALB/c mice were investigated. Methods: Groups of 6-8 weeks old female BALB/c mice were infected with 103, 104 or 105 PFU of H7N9 human isolate, A/ Anhui/01/2013 (AH1) and A/ZheJiang/DTID-ZJU01/2013. For comparison, 2009 pandemic H1N1 virus (A/HK/415742/09), and a recombinant virus rg-PR8-H7-N9 which contains HA and NA gene from ZJ1 virus and the 6 internal genes from A/ PR/8/34 (H1N1) were also studied. At 7, 14 or 28 days after virus infection, serum samples from infected mice were examined for hemagglutination inhibition (HI) antibody and microneutralization (MN) antibody. Results: After infection with AH1, all the mice developed serum hemagglutination inhibition (HI) antibody (GMT= 40-72.5) at day 14 post infection, the titer increased further at day 28 p.i. (GMT=171.5-201.6). However, most of the infected mice had no detectable neutralizing antibody even at day 28 p.i. by standard microneutralization (MN) assay. To study whether the lack of serum neutralizing antibody was also occurred in other H7N9 virus infection, 105 PFU of ZJ1 were inoculated to BALB/c mice, similar pattern of HI response was observed, but no detectable MN antibody. From these mouse anti-sera, low level of neutralizing activity could be detected by fluorescent focus microneutralization (FFMN) assay, but passive transfer of the antisera to infected mice showed no protection effects. Through comparison, we found that the pattern of antibody response to H7N9 infection is dramatically different from that of 2009 pandemic H1N1 virus infection, A/HK/415742/09 H1N1 induced robust high titer of HI and MN antibody responses at day 14 p.i., and the anti-sera protected mice from lethal infection when passive transferred to the same strain of virus infected mice. To study whether the observed antibody response in H7N9 infection is solely related to the proposed low frequency of T cell epitopes on its surface HA proteins, a recombinant influenza rg-PR8-H7-N9 virus was studied in mice. The results showed that rg-PR8-H7-N9 infection induced significantly stronger serum antibody responses. MN antibody titer reached GMT of 121.3 at day 14 p.i, which further increased to GMT of 278.5 at day 28 p. i.. The MN titers were significantly higher than that induced by wild type H7N9 virus, ZJ1. ZJ1 infected mice only produced very low MN antibody at day 28 p.i, with 2/10 mice had titer of 1:40, 7/10 mice had a titer of 1:20 (GMT=20). These results suggest that the internal genes in the H7N9 virus may also affect the antibody responses.Biography
Anna J X Zhang is a Research Assistant Professor, Department of Microbiology at the University of Hong Kong. Her research interests are pathogenesis of influenza virus, antiviral therapy for influenza virus infection, epidemiology of emerging infectious disease. She has published more than 25 papers in reputed journals.
Email: zhangajx@hku.hk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi