Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
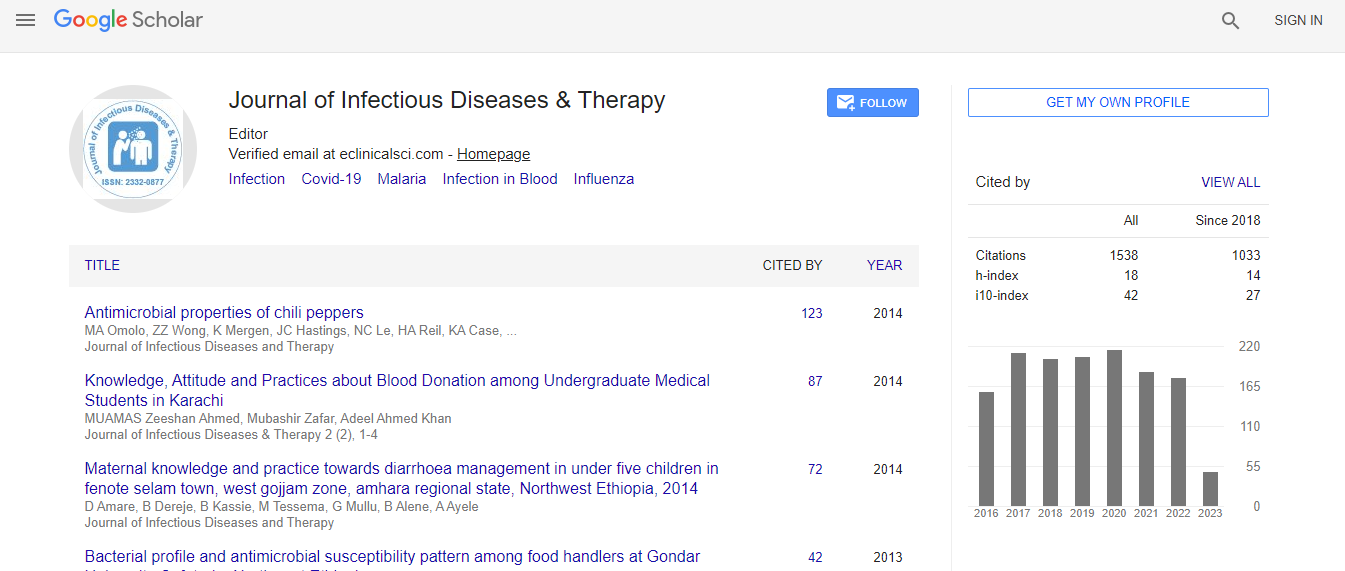
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Globalization of the unmet need of new antibiotics
Joint Event on 2nd World Congress on Infectious Diseases & International Conference on Pediatric Care & Pediatric Infectious Diseases
Glenn S Tillotson
Cempra Pharmaceuticals, USA
Keynote: J Infect Dis Ther
Abstract
Bacterial resistance to antibiotics is an escalating problem. There are significant efforts expanded in the battle to combat this problem. Recent infectious diseases have hit the media especially viral infections such as Ebola, Zika and currently Yellow Fever. The dissemination of these infections is especially worrying but we seem to have played down the bacterial diseases. However, with increasing travel and the growing crisis of refugees it is obvious that the transfer of resistant bacterial infections is highly likely or under-appreciated. Recent examples include azithromycin resistant Shigella sonnei infections; NDM-1 Klebsiella pneumoniae and other pathogens were from overseas. Additionally resistant infections may transfer within a country where there may be marked susceptibility differences. So what may be the implications of this situation? Companies both small and large are developing antibiotics to combat this issue are faced with multiple regulatory processes. These can be challenging both in terms of completion and in terms of costs. As these issues become more global, there needs to be a mechanism by which a streamlined development process applied so each country or region does not need to repeat or require their own unique evaluations to approve a new antibiotic. The clock is ticking and we are running out of options and as we travel more this can only get worse.Biography
Glenn S Tillotson has over 30 years pharmaceutical experience in pre-clinical and clinical research, commercialization, medical affairs, scientific communications including publication planning strategic drug development, life cycle management and global launch programs. He has been instrumental in the development and launch of ciprofloxacin, moxifloxacin, gemifloxacin, fidaxomicin and several other agents. He is a SVP of Medical Affairs where he is preparing for the launch of solithromycin for community acquired bacterial pneumonia. He has published more than 170 peer-reviewed manuscripts and is on several journal Editorial Advisory Boards including the Lancet Infectious Disease, eBioMedicine, Expert Reviews in Anti-infective Therapy and F1000.
Email: gtillotson@cempra.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi