Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
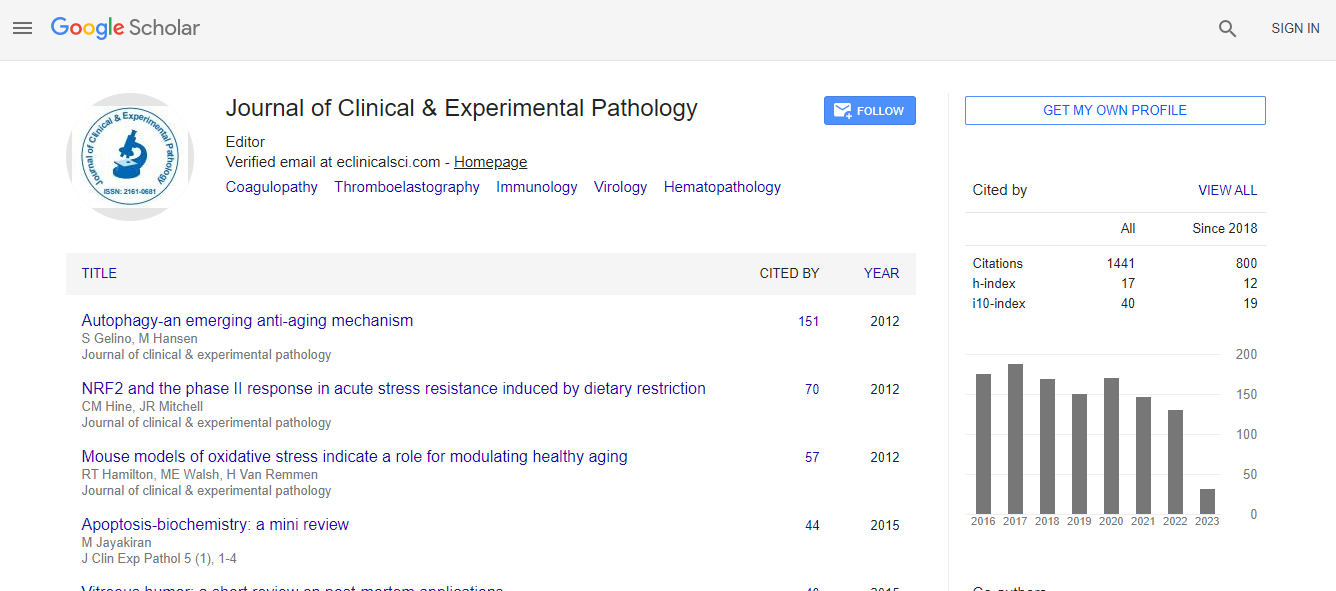
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Genomic analysis of intra-tumor heterogeneity unveil cancer evolution
9th World Digital Pathology & Pathologists Congress
Koshi Mimori
Kyushu University Beppu Hospital, Japan
Posters & Accepted Abstracts: J Clin Exp Pathol
Abstract
The ultimate goal of our study is to improve the clinical outcomes for patients with malignancies, particularly of the gastrointestinal (GI) tract; however, such tumors are often refractory to treatment. One of the main causes of this intractability is the genomic heterogeneity of cancer, which complicates the development of genetically based therapeutics. We have proposed two approaches to clarifying when or how malignant cells acquire genomic heterogeneity. One addresses inter-tumor heterogeneity and the other addresses intra-tumor heterogeneity. In order to assess genomic heterogeneity in samples from human cancers, we have applied next generation sequencing and super-computational analysis with simulation. We believe that understanding the development of genomic heterogeneity in cancer cells can help elucidate the evolution of malignancy and may suggest interventions to eliminate the progression of malignancy and ultimately improve prognosis for affected patients. Our work to date has focused primarily on characterizing inter-tumor genomic heterogeneity in cases of esophageal cancer, which is the most intractable malignancy among GI cancers. Based on mutational signatures in 144 cases of Japanese esophageal squamous cell carcinoma (ESCC), the diverse patients could be clustered into three risk-factor subtypes: (1) Environmental factors, i.e., drinking and smoking, (2) polymorphisms in the aldehyde dehydrogenase 2 (ALDH2) gene and (3) Polymorphisms in the cytochrome P450 2A6 (CYP2A6) gene. To address the analysis of genomic variability within a primary tumor, we employed a novel approach, analyzing multiple regions within a tumor to identify genomic heterogeneities and to determine as much as possible about the order in which they arose. Computational analysis with simulation allowed us to deduce the evolution of a tumor├ó┬?┬?s heterogeneity. In this study, we dissected multiple samples from mutually exclusive tumor regions of nine cases of colorectal cancers and interrogated them with exome sequencing, gene copy number analysis, DNA methylation arrays and microarray-based gene expression studies. In each case, we were able to identify ├ó┬?┬?founder├ó┬?┬Ł mutations, which were detected in all regions sampled and progresser mutations that were found in some regions but not all. We found that founder mutations were associated with aging. At the gene copy number level, focal amplifications were observed to occur more frequently in founder mutations, while focal deletions were more common in progresser mutations. Epigenetic annotation indicated that CpG-island hyper methylation was an age-related, early event in tumor development and that global hypomethylation was a feature of tumor progression. This multidimensional survey, coupled with computational simulation, revealed that most intra-tumor genomic heterogeneity is likely to be generated by ├ó┬?┬?neutral evolution├ó┬?┬Ł not by ├ó┬?┬?Darwin's Theory of Evolution├ó┬?┬Ł. In other words, most mutations observed in a tumor arise incidentally and are neutral in terms of tumor progression. Such neutral mutations are called ├ó┬?┬?passenger mutations├ó┬?┬Ł, to distinguish them from the ├ó┬?┬?driver mutations├ó┬?┬Ł that actively promote tumor cell proliferation. This may suggest that the refractoriness to treatment observed in some tumors may be caused when a therapeutic treatment has the unintended effect of converting one or more passenger mutations to driver mutations, thereby conferring therapeutic resistance.Biography
Email: pa19107@me.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi