Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
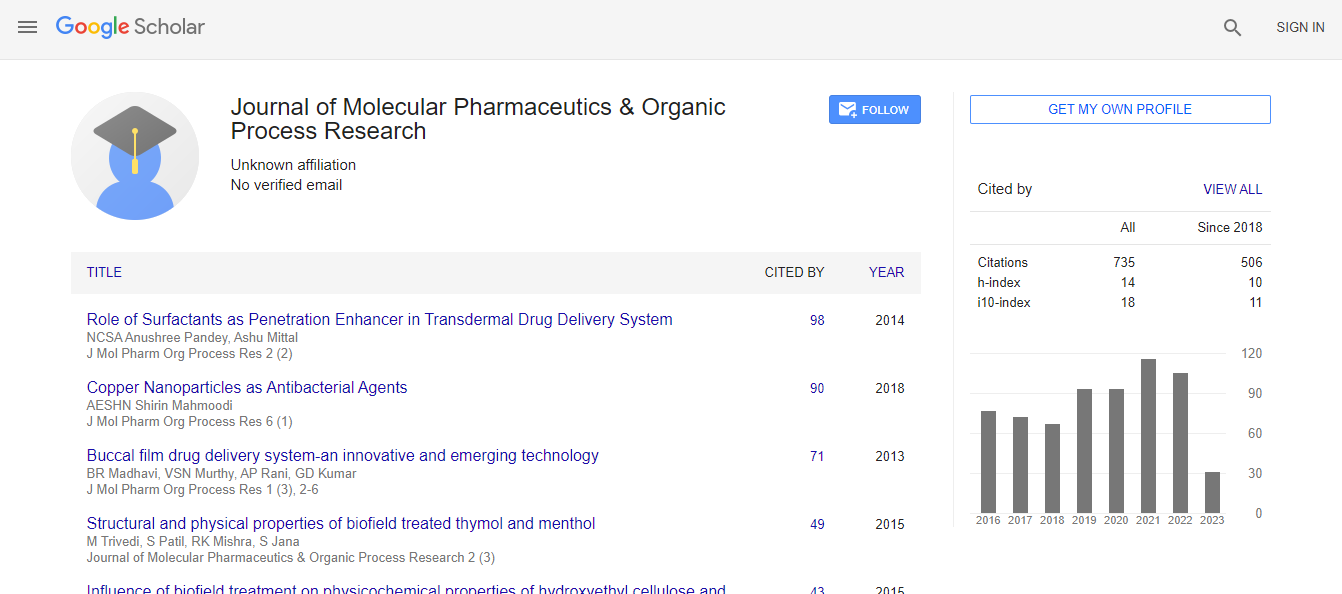
Google Scholar citation report
Citations : 1057
Journal of Molecular Pharmaceutics & Organic Process Research peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Share This Page
Formulation of an extemporaneous preparation for pediatric use: Suspension of Captopril at 1 mg/ml
2nd International Conference on Pharmaceutical Formulations and API
Benaziz Ouarda* and Djenadi lila
Blida University, Algeria
ScientificTracks Abstracts: J Mol Parm Org Process Res
Abstract
The pharmaceutical development and marketing of a drug is a very complex, long and regulated process. The range of drugs pediatric use is very restricted, as these products are expensive, unprofitable to develop. In the context of dose adjustment, pediatric preparations are the more often made in the form of capsules. The child must benefit from appropriate treatment. Pediatric preparations oral liquids facilitate dose adjustment and easy administration by the hospital staff. The objective of this work is the formulation of a suspension vehicle or matrix of liquid excipient for the adaptation of dosage in pediatrics, Application of the formulated vehicle to an active principle. To meet the need for dosage adjustment in pediatrics, we have proceeded to formulate a versatile stable suspension vehicle which will be used for dosage adjustment. Several formulation tests have allowed establishing several qualitative and quantitative formulas of vehicles for suspension. A sweetener and flavorings have also been added to improve the palatability and consequently the compliance of the treatment. Several parameters were checked: density, pH, rheological study. The vehicle prepared was used for the preparation of a suspension of captopril at 1 mg/ml. A stability study was carried out for 60 days. The pH and the final viscosity of the vehicles prepared meet the objectives set in the departure. The physical stability of the vehicles as well as the rheological behavior has been considered satisfactory for several formulations. Suspensions containing the principle active ingredient showed a pH within the acceptability range of 4-5. The stability of the suspensions of active ingredients is conditioned by the stability of the vehicles. Chemical stability was also assessed by spectrophotometry Visible UV. The rheological study demonstrated the shear-thinning behavior of certain formulas. At the end of this work, we ended up with the formulation of a vehicle for extemporaneous preparation also called matrices of liquid excipients in the shear-thinning behavior. The latter was applied to the preparation of a suspension of captopril at 1 mg/ml. This work was motivated by the lack of formulations suitable for pediatric use. In addition, captopril is widely prescribed by pediatricians especially for children with heart disease congenital. Thus, the presence of pharmacists at the level of pharmacies hospitals would enable the adoption of safe preparation practices and scientifically proven.Biography
Benaziz Ouarda is an Associate Professor With experience in pharmaceutical research and development. Currently she is practicing at the university hospital center.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi