Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
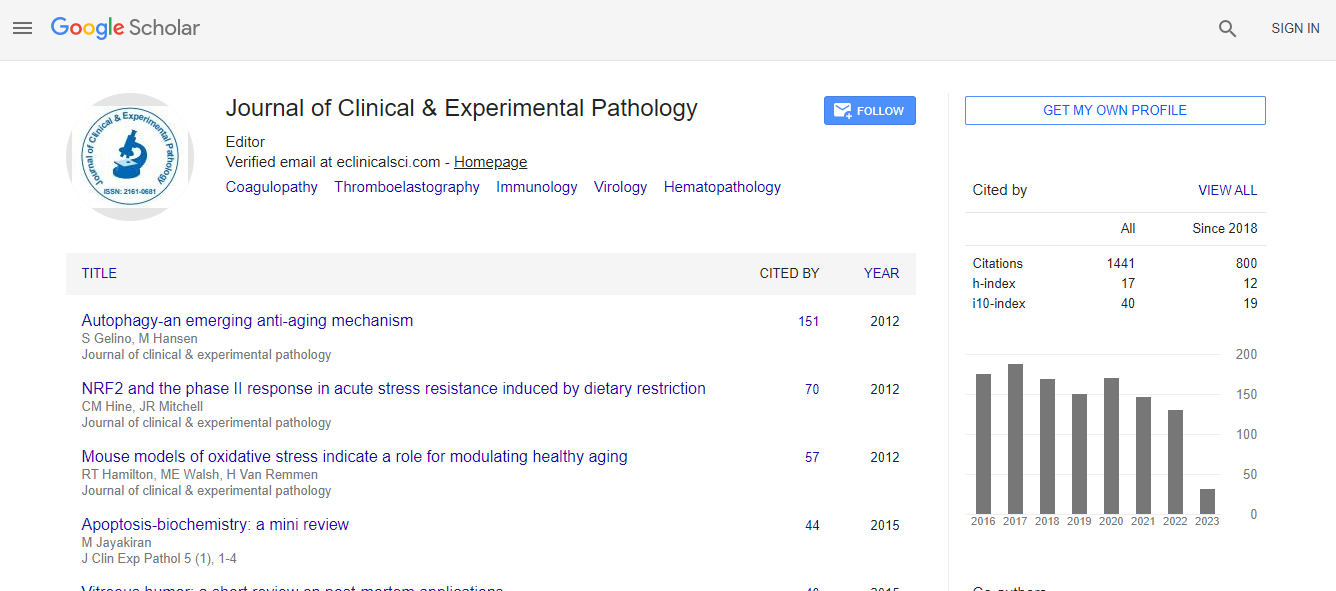
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
Extreme Response to Immunotherapy in an Estrogen Receptor Positive Breast Cancer: A Case Report
3rd International Conference on Breast Pathology and Cancer Diagnosis
Rebecca Fitzpatrick
Rush University Medical Center, Chicago, USA
ScientificTracks Abstracts: J Clin Exp Pathol
Abstract
Introduction: Currently, immune checkpoint inhibitors are not approved by the FDA for HR-positive breast cancer, although an extreme response was seen in the case below. Case: The patient is a 72 year old, woman who was diagnosed in 1996, at age 48, with stage T2N2aM0, ER-positive infiltrating ductal cancer in her left breast. She completed 4 cycles of doxorubicin and cyclophosphamide and she received adjuvant tamoxifen, until 2004, then was switched to letrozole, which was completed in April, 2011, thus completing 15 years of endocrine treatment. She recurred in 2017 with pleomorphic invasive lobular carcinoma, ER 5%, PR 0%, and HER2 not amplified by FISH. She eventually progressed through 5 lines of treatment. The 2/2019 biopsy specimen was sent for next generation sequencing. The tumor was found to have a high Mutational Burden (MB) (21 m/MB, 96th percentile for breast cancer). Pembrolizumab was provided on compassionate plea from the manufacturer in 3/2019. She had a complete response with resolution of severe brachial plexopathy pain. Discussion: Pembrolizumab is associated with good outcomes in cancer other than breast cancer. In these cancers, a higher mutation burden is associated with response. The patient in this case had a high tumor MB, which prompted treatment with pembrolizumab. This case shows the importance of next generation sequencing and PD-L1 staining, enabling the use of immune checkpoint inhibitors as a possible treatment option for HR-positive breast cancer if the tumor has a high tumor MB and/or expression of PD-L1 in TILs.Biography
Rebecca Fitzpatrick was under the Department of Internal Medicine, Division of Hematology, Oncology and Cell Therapy, Rush University Medical Center, USA.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi