Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
42nd International Conference on Dentistry & Dental Marketing
San Francisco, USA
11th International Conference on Complementary & Alternative Medicine
Zurich, Switzerland
9th World Conference on Nursing Education & Nursing Practice
Toronto, CanadaGoogle Scholar citation report
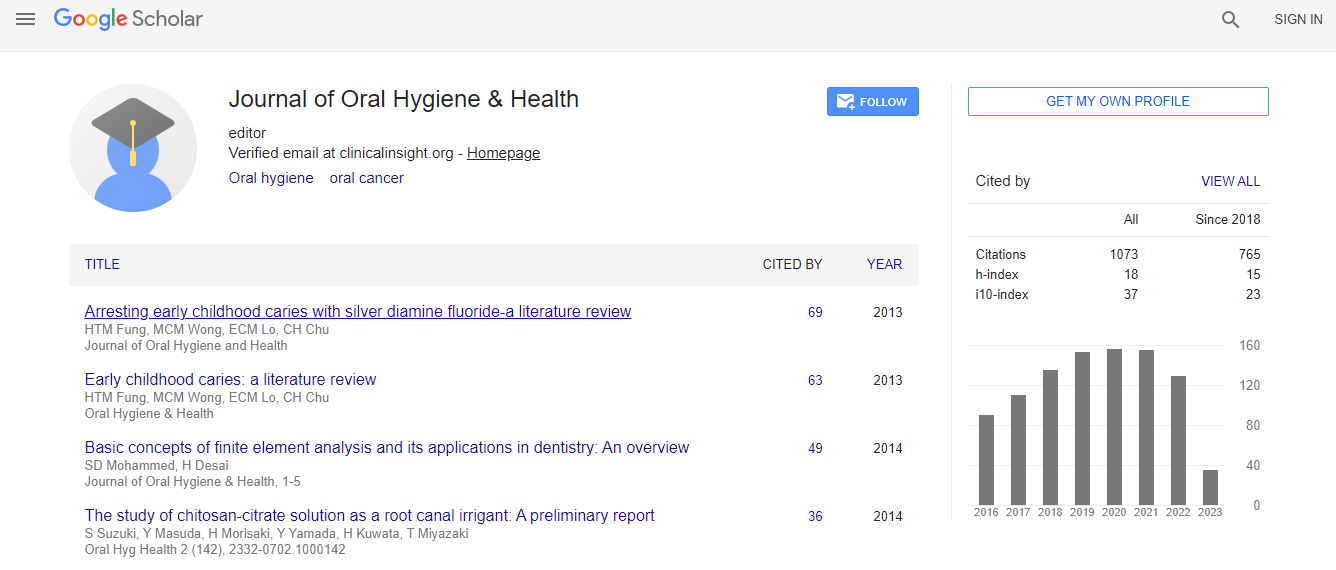
Citations : 1073
Journal of Oral Hygiene & Health received 1073 citations as per Google Scholar report
Journal of Oral Hygiene & Health peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Evaluation of in vitro biofilm removal with l2% and 10% sodium hypochlorite
Annual Congress on Endodontics, Orthodontics, Prosthodontics and Dental Implants
Seyedeh Zahra Rahmani, Mohammad Smiee, Seyedeh Paria Rahmani, Look Vander Sluis and Ferananda Hoffmann Busanello
Tabriz University of Medical Sciences, IranUniversity of Groningen, NetherlandsFederal University of Rio Grande, Brazil
Posters & Accepted Abstracts: J Oral Hyg Health
Abstract
Introduction & Aim: Biofilms are communities of microorganisms attached to a surface and embedded in a matrix of polysaccharides and proteins forming a slimy layer. Oral bacteria have the capacity to form biofilms on distinct surfaces. Bacteria also form dense colonies on root canal walls and features like isthmuses and lateral canals. Microbial communities in biofilms are remarkably difficult to eradicate with antimicrobial agents for reasons that have yet to be adequately explained. Studies have shown that sodium hypochlorite (NaOCl) is the most effective anti-microbial irrigant used during endodontic treatment. The aim of this study is to evaluate the structure of biofilms and presence of EPS before and after the use of NaOCl 2% and 10%. Materials & Methods: Dual species biofilms of Streptococcus oralis J22 and Actinomyces naeslundii T14VJ1 were grown under statical conditions and in a Constant Depth Film Fermenter (CDFF). Biofilms grown in the CDFF mimic better the basal layer of an oral in vivo biofilm. For the statical conditions, a confined space was created over saliva coated dentin discs with supply of 20 ml of modified BHI each 24 h for 4 and 10 days. For the CDFF, saliva coated hydroxyapatite discs biofilm was grown for 96 h at 37 oC under continuous supply modified BHI at a rate of 45 ml/h. The system was equipped with 15 sample holders and each sample holder contained 5 saliva coated hydroxyapatite discs, recessed to a depth of 250 mm. After growing the biofilms NaOCl 2% and 10% were applied for 60 s and 300 s for removing the biofilm. Optical Coherence Tomography (OCT) was used for high-resolution, real-time imaging of a three-dimensional structure of the biofilm. Confocal Laser Scanning Microscopy (CLSM) was used to visualize the biofilm matrix, structure and condition of bacteria (LIVE/DEAD staining). Results: In the statical biofilm group, OCT images showed reduction of biofilm thickness after applying the NaOCl 2% and 10% and there was a very fluffy structure observable. In the CDFF group, OCT images showed bubble formation in the biofilm after using NaOCl 10%, but the irrigant did not reduce the thickness of the biofilm or on its consistency. The bubble formation was also observed in CLSM images. The CLSM showed reduction of the biofilm structure but mostly living bacteria were found in the remaining biofilm. Conclusion: Due to our study our simple irrigation methods are not efficient enough for biofilm removal and we suggest to use irrigants in several times with increased applying time to achieve better biofilm removal and better treatment results.Biography
E-mail: rzahra75@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi