Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
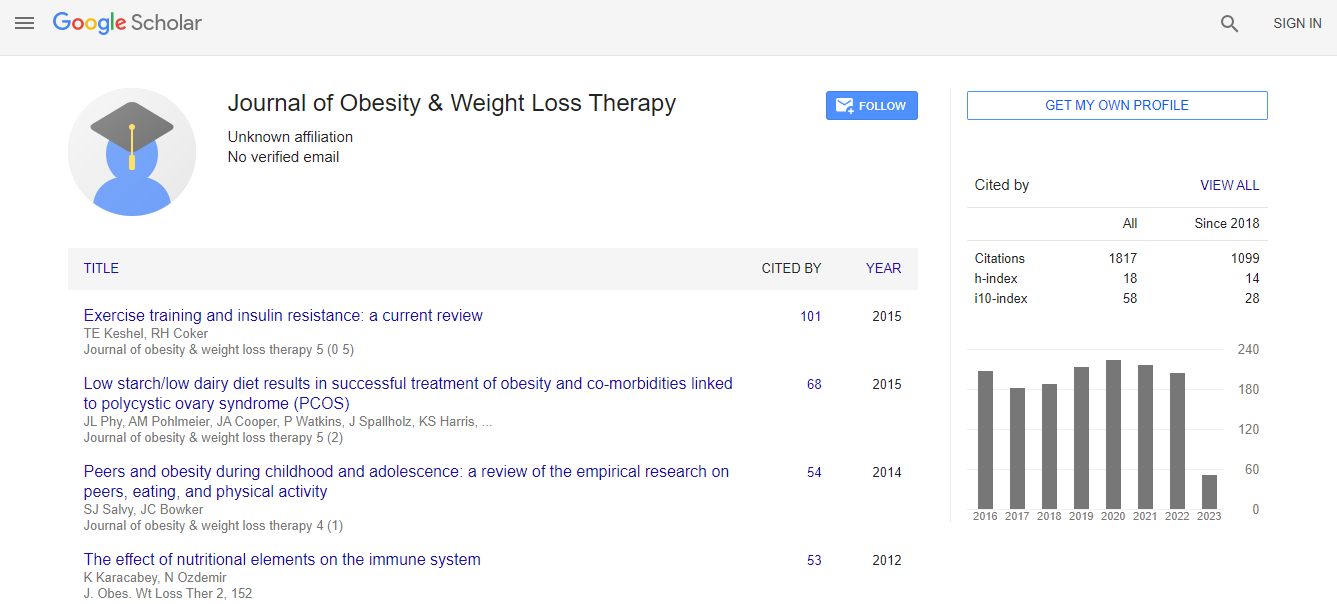
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Epigenetic regulation of IGF-IGFR signaling by H19 non-coding RNA in adult stem cells
3rd International Conference and Exhibition on Obesity & Weight Management
Linheng Li
University of Kansas Medical Center, USA
ScientificTracks Abstracts-Workshop: J Obes Weight Loss Ther
Abstract
Insulin growth factor (IGF) signaling plays a critical role during development and homeostasis of adult tissues. There is compelling evidence that adult stem cells exist in an active (enriching proliferating) and quiescent (functioning as a reserve population) state. Earlier studies from our laboratory revealed differential expression of imprinting genes, including H19 locus, in these two populations in the blood-forming (hematopoietic) system. These observations led us to hypothesize that the epigenetic state of imprinting genes can influence stem cell state and the related function. To test this hypothesis, we used a genetic approach to delete DNR Methylation Region (DMR) in the H19 locus (H19fx/+Ã?Â?DMD) from female or male alleles respectively. Unlike paternally inherited deletion (H19 Ã?Â? DMD), maternally inherited deletion resulted in phenotypic reduction in quiescent hematopoietic stem cells (HSCs) and increase (at early stage) in proliferating HSCs, accompanied with compromised function of HSCs. These data suggest that the epigenetic state of imprinting genes regulates stem cell state and function. Deletion of the DMD region was accompanied by a decrease in H19 gene expression and reciprocal upregulation of Igf2, resulting in activation of the Igf2-Igfr1 pathway, thus leading to increased activation, proliferation, and eventual exhaustion of HSCs. Mechanistically, maternal-specific H19-DMR deletion led to an increased translation of Igf1r, which is normally suppressed by H19-derived miR-675. Similarly, genetic inactivation of Igf1r partially rescued the H19-DMR deletion phenotype. Our work establishes a novel role for this unique form of epigenetic control at the H19-Igf2 locus in maintaining adult stem cells via regulating IGF-IGFR signaling.Biography
Linheng Li is best known for using genomics and genetics to study stem cells and their niches in hematopoietic and intestinal tissues. He was among the first to identify the endosteal (osteoblastic lining cell) niche in supporting hematopoietic stem cell (HSC), the first cellular component of niche identified in a mammalian system. Based on the study of different HSC subpopulations, he was also among the first to propose co-existing quiescent (as a reserve pool) and active (primed for action) adult stem cells in the same tissue in mammals. His recent work shows a critical role of epigenetic regulation of H19-Igf2 imprinted locus in controlling quiescent versus active states of HSCs.
Email: lil@stowers.org

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi