Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
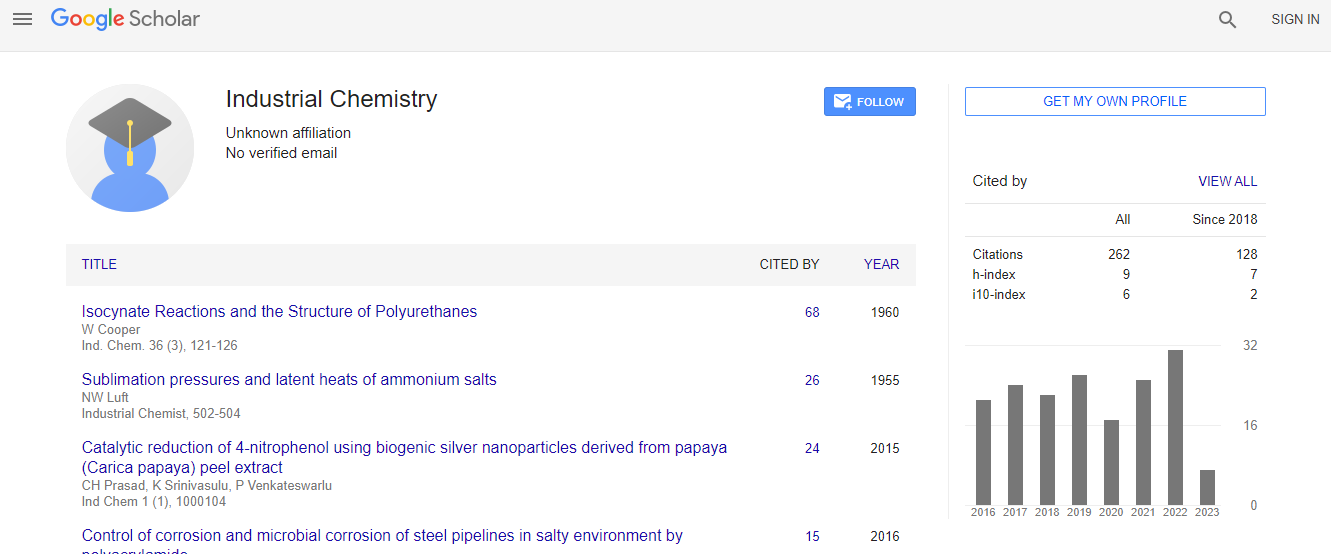
Google Scholar citation report
Citations : 262
Industrial Chemistry received 262 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Recommended Journals
Related Subjects
Share This Page
Environment-induced conformational and functional changes of L-2-haloacid dehalogenases
International Conference on Industrial Chemistry
Yayue Wang
Chinese Academy of Sciences, China University of Chinese Academy of Sciences, China
ScientificTracks Abstracts: Ind Chem
Abstract
Enzymes are widely used as biocatalysts in various important industrial processes because of their unique features such as substrate specificity, rate acceleration, regio-, chemo-, and stereoselectivity. Understanding the environmental effects on the structure-function relationship of enzyme is significant for evaluating the enzymatic activity during application. Up to now, L-2-Haloacid dehalogenases have been highly studied for the biochemical and structural characterization, however, no information was available regarding environmental effects on the structure-function relationship. Here, circular dichroism spectroscopy (CD) was used to investigate the correlation between changes on the conformation and the function of L-2- haloacid dehalogenase (HadL AJ1) from the Pseudomonas putida induced by the environmental factors. Decreased �±-helix and increased �²-sheet contents were observed in the structure of HadL AJ1 along with activity losses caused by pH, temperature and inhibitors. Regardless of which factor above-mentioned existed, more than 65.0% of HadL AJ1 activity could be remained if its �±-helix content was over 12.0%. The maintenance of �±-helical structure in HadL AJ1 was indispensable to its catalysis, while �²-sheet increase restricts its activity. This study revealed the variation of enzymatic activity due to environmental conditions resulting in structural changes monitored by CD, which contributed to rational modification and was instructive for predicting changes of the enzymatic activity during application.Biography
Yayue Wang is a PhD candidate in Dalian Institute of Chemical Physics of Chinese Academy of Sciences, under the supervisor of Dr. Song Xue. Her project is about characterization of haloacid dehalogenases, focusing on its structure-function relationship.
Email: yayue0841@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi