Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
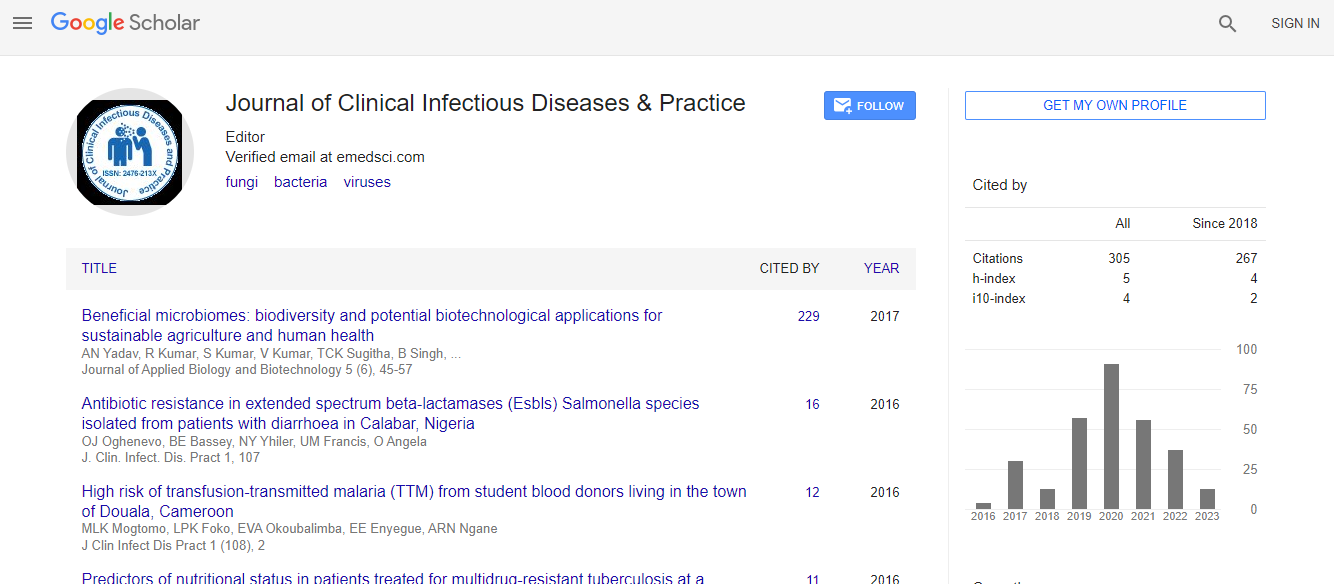
Google Scholar citation report
Citations : 531
Journal of Clinical Infectious Diseases & Practice peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
Ensuring patient centricity in rare disease drug development
9th World Congress on Rare Diseases and Orphan Drugs
Jennifer Mcnary
Fierce advocate, USA
Posters & Accepted Abstracts: J Clin Infect Dis Pract
Abstract
Patient centricity is an often used but not always executed strategy in the current drug development landscape. It is known that patients and their caregivers are key stakeholders and potentially hold the greatest expertise in their own diseases; it is the responsibility of drug makers to enlist the help of these individuals to help ensure a successful pathway from pre-clinical development to the global market. During this presentation author will use her 17 years of experience as a caregiver to two rare disease patients and professional work as an Advocate and Consultant to demystify the concept of ensuring patient centricity in the rare disease drug development space. Participants should expect the following to be achieved: Exploring timelines and methods for seeking patient input into the drug development process at every stage, including endpoint selection, clinical trials, regulatory and commercial development; Learning best practices for working with patient, caregivers and advocacy groups to ensure a mutually beneficial relationship; Identification of ways to support and encourage the patient communities in your disease space; Development of strategies to tackle access issues within patient communities both pre and post drug approval.Biography
E-mail: Jenn@JMcNaryconsulting.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi