Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
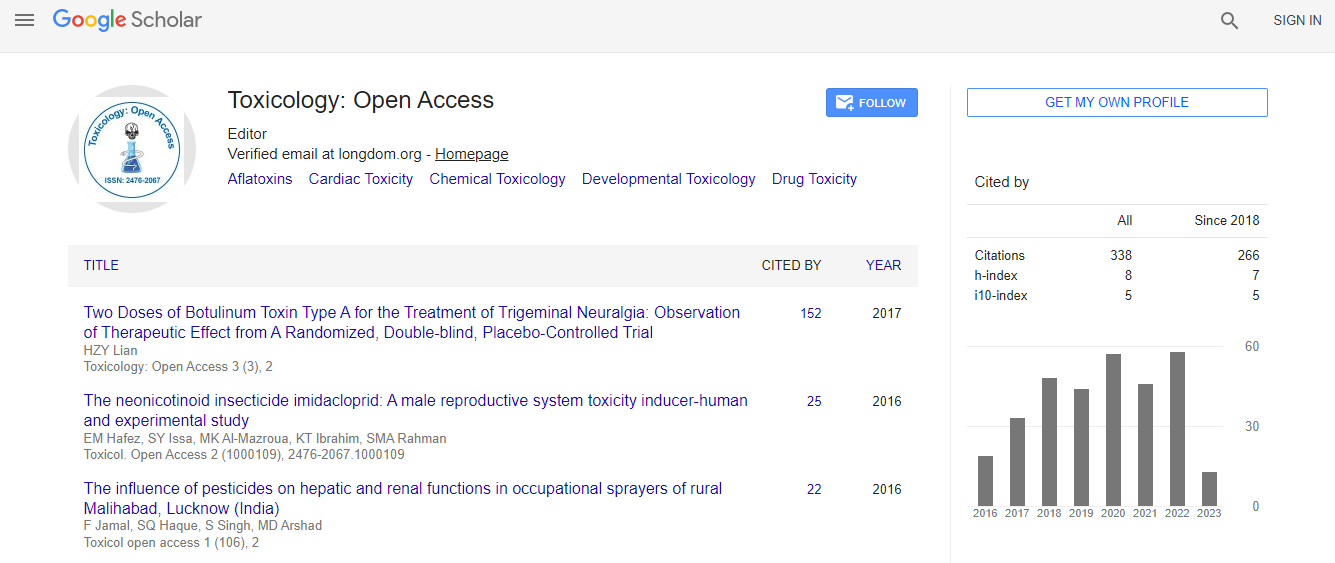
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Endocrine disruption and antimicrobials
8th World Congress on Toxicology and Pharmacology
Jirova Dagmar
National Institute of Public Health, Czech Republic
Keynote: Toxicol Open Access
Abstract
Endocrine disruptors are hormonally active substances of natural or synthetic origin affecting the endocrine (hormonal) systems of humans. Such compounds can be found in chemical groups like steroids, cyclic hydrocarbons, phenols, flavonoids, phtalates, parabens or toxic metals. They are used as antimicrobials, biocides, plasticizers, surfactants, UV filters or fire retardants. They may be released from consumer products, e.g. cosmetics, toys, food packaging materials, household products, medical devices and other products of industry or agriculture. In the EU, they are banned for consumer products. Recently regulated CMR substances from the group of Antimicrobials/Preservatives (biocides) comprise: Chloracetamide (Reprotox. Cat. 2), Phenol (Mutagenic Cat. 2), Nonylphenol (Reprotox. Cat. 2), Parabens (pentyl-, phenyl-, benzyl- for absent data on reprotox.), Ketoconazole (Reprotox. Cat. 1B), Boron compounds (Reprotox. Cat. 1B), Formaldehyde (Carcinogenic Cat. 1B), Polyaminopropyl Biguanide-PHMB (Mutagenic Cat. 2). Significant reprotoxic effect has been proved in the past namely for distinct bisphenols (Reprotox. Cat. 2) or phthalates (Reprotox. Cat 1B or Cat. 2) which were subsequently banned. However, the production of analogous compounds is increasing underlining the necessity to test their safety including reprotoxicity. The European Commission's general policy is the use of alternative toxicological methods in vitro instead of conventional tests on vertebrates. Available methods in vitro to detect endocrine disruption are: OECD TG 455/457ΓΆΒ?Β?Estrogen Receptor Transactivation Test Method, OECD TG 456ΓΆΒ?Β? Effects on Steroidogenesis, OECD TG 236ΓΆΒ?Β?ZFET, zebrafish embryo epigenetic assay, MCF-7 cell proliferation assay, Xenoscreen YES/YAS yeast assay. Results of a pilot study to prove applicability of methods in vitro to detect reprotoxicity are presented.Biography
Jirova Dagmar, MD, PhD, graduated and received her PhD at the Charles University in Prague. Her professional specialization is in Dermatotoxicology and Immunotoxicology, focused on safety assessment of cosmetics and other consumer products. She is holding a position of the Head of Centre of Toxicology and Health Safety at the National Institute of Public Health, Prague, Czech Republic. She is the author of more than 200 publications in scientific journals, proceedings and monographs, posters or articles and publications for the public. She was the Principal Investigator in number of research projects in the field of alternative toxicological methods for evaluation of health risks of chemicals and consumer products.
Email: jirova@szu.cz

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi