Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaGoogle Scholar citation report
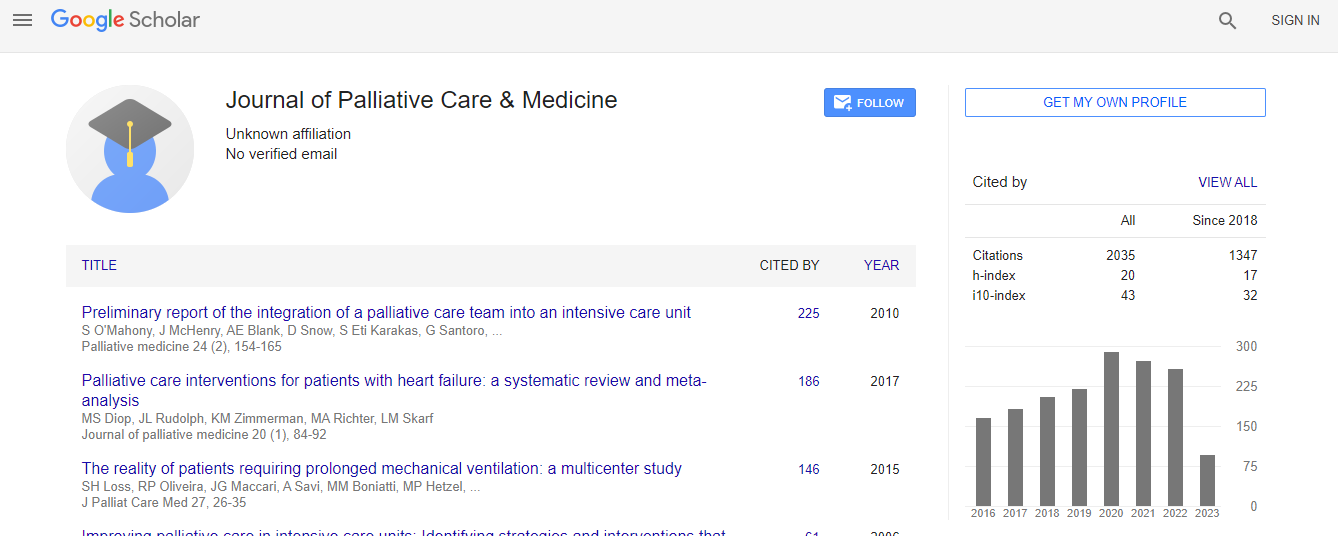
Citations : 2035
Journal of Palliative Care & Medicine received 2035 citations as per Google Scholar report
Journal of Palliative Care & Medicine peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Efficacy of the addition of duloxetine for neuropathic cancer pain refractory to opioids and gabapentinoids: A multi-institutional, randomized, double-blinded, placebo-controlled trial (JORTC Pal 08 (trials in progress)
5th World Congress on Hospice and Palliative Care
Hiromichi Matsuoka
University of Technology Sydney, AustraliaKindai University, Japan
Posters & Accepted Abstracts: J Palliat Care Med
Abstract
Management of cancer patients suffering from neuropathic pain refractory to opioids and gabapentinoids remains an important challenge. Duloxetine is one of the choices after first-line treatment fails. The efficacy of duloxetine has been reported in non-cancer patients and in chemotherapy-induced polyneuropathy, but no randomized clinical trials have examined its effects on neuropathic cancer pain refractory to first-line treatment. A multi-institutional, prospective, randomized, double-blind, placebo-controlled, two-parallel trial is planned. The inclusion criteria are adult cancer patients suffering from neuropathic pain refractory to opioids and gabapentinoids, patients with a Numerical Rating Scale (NRS) pain score of 4 or higher and patients with a total Hospital Anxiety and Depression Scale (HADS) score of less than 20. Patients with chemotherapy-induced peripheral neuropathy are excluded. The study will take place at 14 sites across Japan. Participants will be randomized (1:1 allocation ratio) to a duloxetine intervention group or a placebo control group. Evaluations will be made at baseline (T0 randomization), day 0 (T1), day 3 (T2) and day 10 (T3). The primary endpoint is defined as the difference in NRS score for pain intensity (average over the previous 24 hours) at T3 between the duloxetine and placebo groups. The enrolment started in July 2015. At the time of manuscript submission (November 2017), more than 95% of patients have participated. We thus expect to complete the recruitment by December 2017. Treatment of neuropathic pain in cancer patients represents an area of high unmet medical need. To our knowledge, there has been no randomized study of the analgesic efficacy of duloxetine in patients with neuropathic cancer pain refractory to opioids and gabapentinoids. This study of duloxetine in neuropathic pain refractory to opioids and gabapentinoids will be the first registered trial of therapy for this condition.Biography
Hiromichi Matsuoka has expertise in evaluation and passion in improving the health and well-being. He is currently working as a Visiting Professor in University of Technology Sydney and preceding his research in patients with cancer pain. He has built his backgrounds as an Anesthesiologist, Physician of Psychosomatic Medicine and Palliative Care Doctors after years of experience in research, evaluation and teaching both in hospitals and educational institutions.
E-mail: matsuoka_h@med.kindai.ac.jp

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi