Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaGoogle Scholar citation report
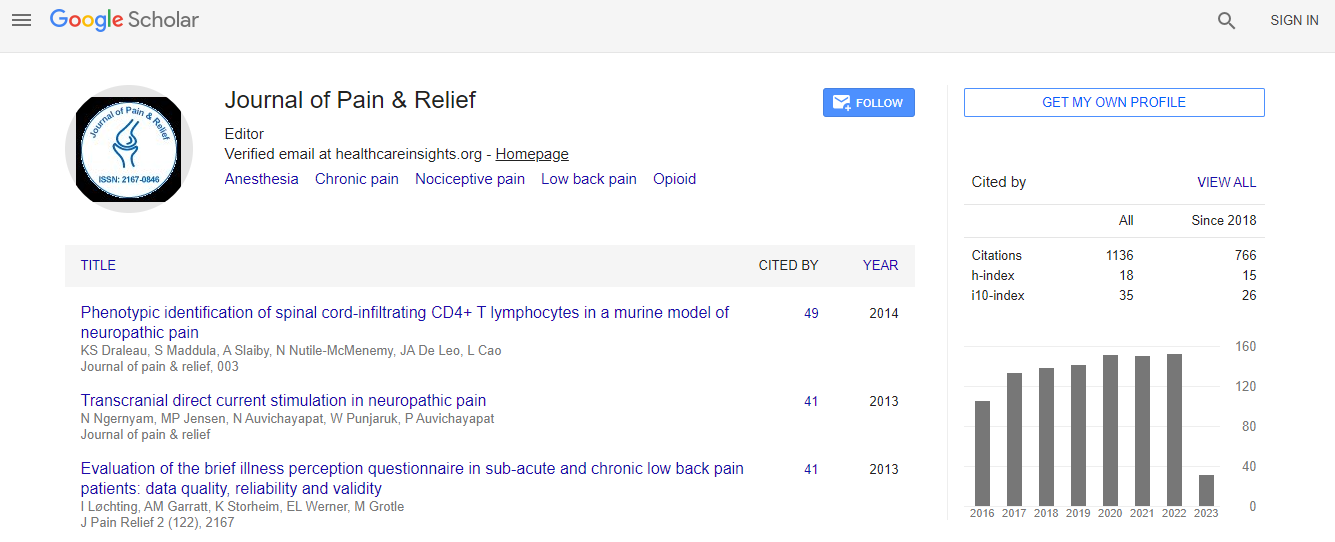
Citations : 1583
Journal of Pain & Relief received 1583 citations as per Google Scholar report
Journal of Pain & Relief peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Cosmos IF
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Efficacy of repetitive transcranial magnetic stimulation for the treatment of fibromyalgia
7th International Conference and Exhibition on Pain Research and Management
Nilson N Mendes Neto, Jessika Maia, Waleska J do N Freitas, Marcelo Rodrigues Zacarkim, Julieli N Teixeira and Levi H Jales Jr
University of California, USAHUOL, BrazilCentro Clinico da Dor, BrazilHarvard Medical School, USA
Posters & Accepted Abstracts: J Pain Relief
Abstract
Background: rTMS is a neuromodulation technique that has been used to treat FM. Data regarding to its efficacy and safety is lacking. Objective: To assess the efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) for the treatment of fibromyalgia (FM). Methods: Open-label uncontrolled trial where 17 subjects diagnosed with FM were enrolled. The recruitment period was from January 2015 to May 2017. All subjects received rTMS in the left prefrontal cortex. The sessions were performed in a series of 3 to 5 consecutive days with maximum break of 2 days between the series. A minimum of 10 sessions was required. Parameters used: frequency (10 Hz), cycles of 10 stimuli with pause of 20 seconds between them. 20 minutes was the length of each session. Motor threshold was adjusted according to the acceptance of patients. Side effects, widespread pain, Q of L, depressive symptoms, insomnia and fatigue were assessed after each session. Results: Among the 17 patients, 88.2% were women. Mean sample age of 55.7 years (ranging from 31–81 years). 41.2% reported significant improvement of pain after 3rd rTMS session. Improvement of depressive symptoms was observed after 3rd session in 50% of patients. Improvement of insomnia and fatigue was reported after 3rd session in 52.9% in 35.3% of patients, respectively. Increased quality of life was seen in 47.1% of patients after the 3rd session. Three patients report mild and transient symptoms such as tinnitus and headache. Conclusions: In our experience, rTMS had a significant influence on pain reduction in patients with FM. Plus, it showed to be a suitable option for rapid relief of symptoms since most patients reported relief of widespread pain and psychogenic/ psychosomatic symptoms after the third session. rTMS was well tolerated with minimal adverse effects. Additional studies are needed to determine optimal protocols for the use of rTMS for the treatment of FM.Biography
E-mail: doctornmendes@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi