Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
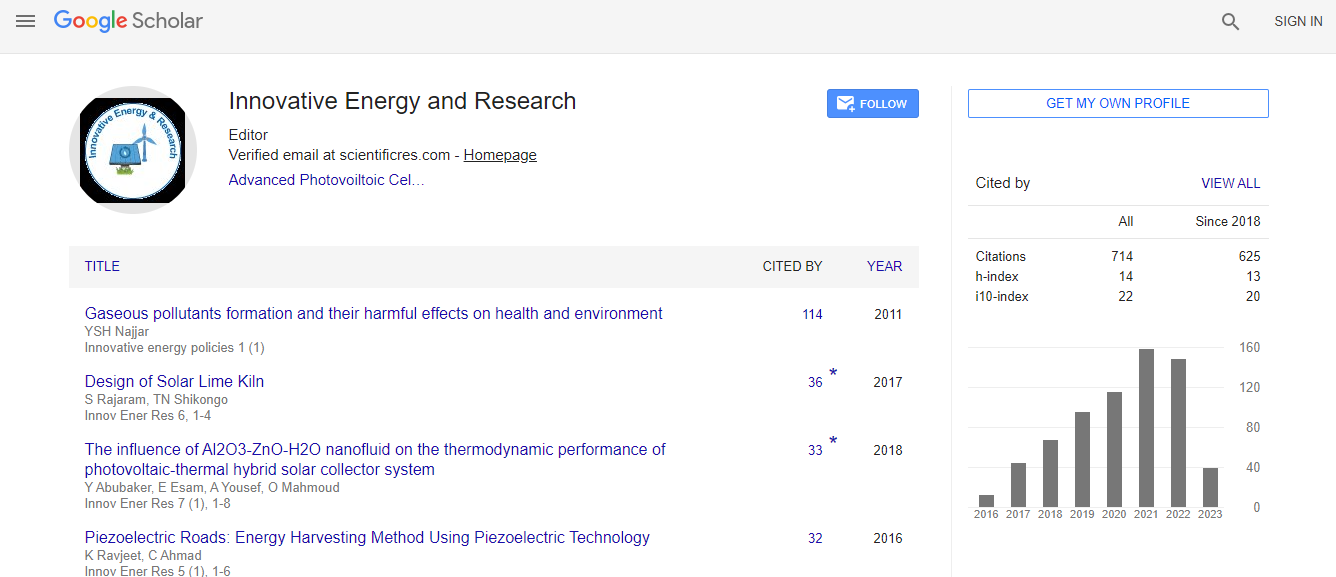
Google Scholar citation report
Citations : 712
Innovative Energy & Research received 712 citations as per Google Scholar report
Innovative Energy & Research peer review process verified at publons
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Discrete Zn, Co bimetallic sites supported on N doped carbon for high performance oxygen reduction reaction catalysis
21st International Conference on Advanced Energy Materials and Research
Yufeng Zhao, Ziyang Lu and Hongguan Li
Shanghai University, ChinaYanshan University, China
Posters & Accepted Abstracts: Innov Ener Res
Abstract
A new design of discrete Zn, Co bimetallic sites supported on N-doped carbon was fabricated through a competitive complexation strategy. Aberration corrected atomic resolution high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) measurements combined with X-ray absorption fine structure (XAFS) reveals the existence and the structure of the Zn-Co bimetallic sites. This Zn-Co dual atom catalysts exhibit significantly improved oxygen reduction catalytic activity compared to single atom catalysts in both acid and alkaline conditions. Density functional theory (DFT) calculations reveal that the enhanced catalytic activity can significantly be attributed to the elongated O-O bond length (from 1.23 Å to 1.42 Å), and thus facilitates the cleavage of O-O bond at the ZnCoN6(OH) sites, showing a theoretical over potential of 0.335 V during ORR process. In-situ XAS study demonstrates that Co serves as the active center during the catalysis. Furthermore, a highly active sulfur (S)-modified Zn, Co-Nx-C-Sy ORR catalyst is also developed. Besides the elongated O-O band length, the S doping can further modify the charges around Zn, Co active center and strengthen the interaction with oxygenated species by decreasing the free energy changes of *O2 + e- + H2O→*OOH + OH- step. The prepared catalysts show promising potential in practical applications in both fuel cell and Zn-air batteries. Particularly, the H2/O2 fuel cell tests based on the Zn-Co atomic pair presents a peak power density of 705 mW cm-2 along with excellent stability.Biography
E-mail: yufengzhao@ysu.edu.cn

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi