Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaGoogle Scholar citation report
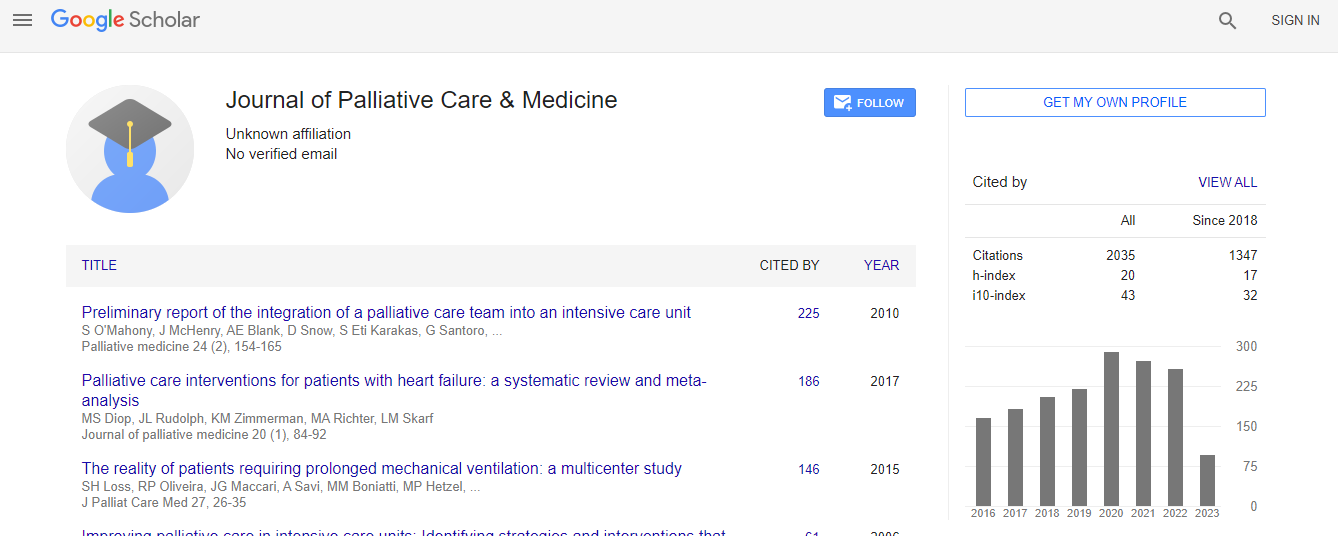
Citations : 2035
Journal of Palliative Care & Medicine received 2035 citations as per Google Scholar report
Journal of Palliative Care & Medicine peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Development of a therapeutic algorithm to guide clinicians considering palliative sedation therapy in pediatric patients at the end of life
International Conference on Hospice & Palliative Care
Justin N Baker, Liza-Marie Johnson and Doralina L. Anghelescu
St. Jude Children├ó┬?┬?s Research Hospital, USA
Posters-Accepted Abstracts: Posters-Accepted Abstracts
Abstract
Background: Despite advances in palliative care a subset of patients experience suffering at the end-of-life refractory to traditional therapy. Palliative sedation therapy (PST) with medications achieving continuous deep sedation (CDS) is well documented in the adult literature as an ethically permissible treatment for intractable suffering. Guidelines for CDS in children are less clear. Objective: Clarify the ethical permissibility of CDS in children at the end of life and develop an algorithm for use in clinical practice. Design/Methods: Identification of patients who received propofol PST within 20 days of inpatient death (2003-2010) and review of the medical record for indicators of pain, suffering, and sedation from 48 hours before PST to the time of death. Based upon this case series we conducted a literature review and developed a clinical algorithm for initiation of PST in children. Results: Three of 192 (1.6%) of children received propofol PST. Use of opioids and supportive medications decreased in 2 cases and in the third case, CDS was effective in relieving other distressing symptoms. Clinical notes suggested improved comfort and rest in all patients. CDS is justified to relieve end-of-life suffering only if the distressing symptoms are refractory to standard palliative management. The indications for PST at the end of life include two core components: the presence of severe suffering refractory to standard palliative management and the primary aim of relief of distress. No ethical contraindication to the use of PST exists in children and numerous case series, including our own, demonstrate its├ó┬?┬? efficacy. We provide an algorithm for clinicians considering PST in a pediatric patient at the end of life. Conclusions: Children with intolerable suffering at the end-of-life should not be allowed to continue with refractory symptoms due to clinician inexperience with this infrequent intervention ├ó┬?┬?of last resort.├ó┬?┬Ł Our algorithm provides a guideline for clinicians and institutions to use in clinical practice.Biography
Email: Justin.Baker@STJUDE.ORG

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi