Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
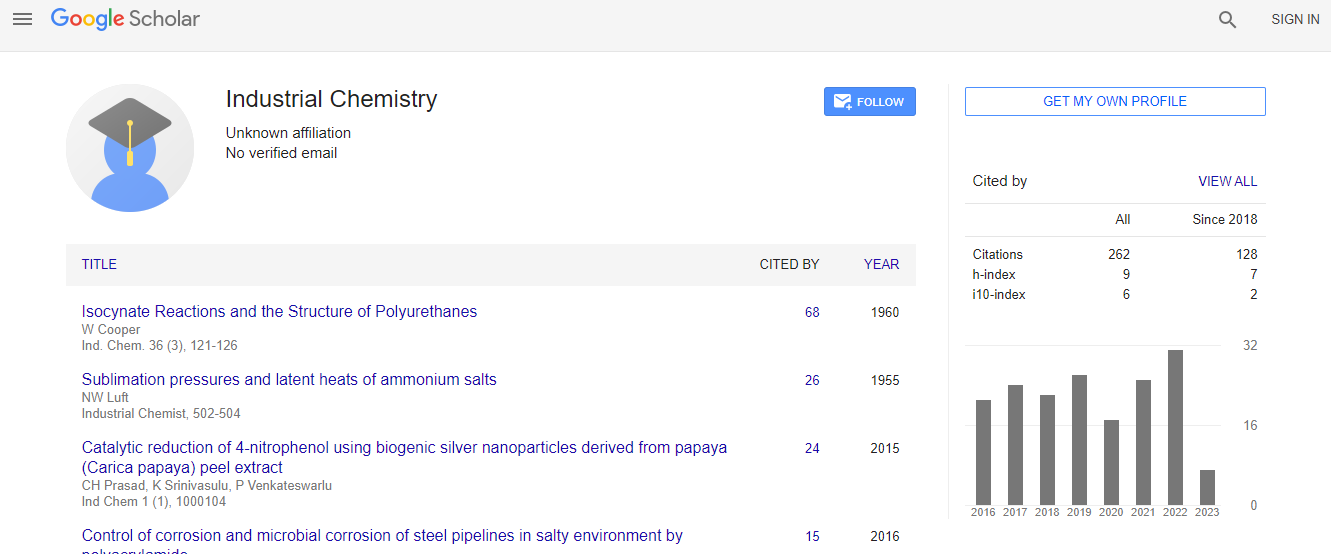
Google Scholar citation report
Citations : 262
Industrial Chemistry received 262 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Geneva Foundation for Medical Education and Research
- Euro Pub
Useful Links
Recommended Journals
Related Subjects
Share This Page
Corrosion inhibition of mild steel in formic acid and citric acid using tamarind (Tamarindus indica) extract
International Conference on Industrial Chemistry
Linus N Okoro
American University of Nigeria, Nigeria
ScientificTracks Abstracts: Ind Chem
Abstract
Organic and inorganic compounds have been widely used as corrosion inhibitors in metal or metallic-alloys. Research has shown that some of the compounds used are toxic and harmful to human health and the environment. Therefore, green chemicals are used as a substitute for the toxic chemicals. In this research, adsorption and thermodynamics of corrosion inhibition on mild steel has been carried out in Citric acid and Formic Acid solutions using Tamarindus indica. The inhibitive properties of ethanolic extracts of Tamarindus indica on mild steel in Formic and Citric acid solutions were investigated using the weight loss techniques. Mild steel was immersed in three different concentrations of the two acids (formic and citric acid) in blank, 0.1 g/100 ml and 0.3 g/100 ml concentrations of the extract. All these three different concentrations were studied within the temperatures of 30oC and 45oC. The results obtained illustrate that the inhibition efficiency increases with increase in concentration and temperature. Increase in inhibition efficiency with increase in temperature suggests the mechanism to be chemisorption. The optimum inhibition efficiency was 78.3 in 3 g/100 ml of citric acid at 45oC (318K). The heat of absorption was found to be positive, hence endothermic. Adsorption studies were carried out using Temkin, Flory- Huggins, and Langmuir isotherms. From the results obtained, the data fits the Langmuir adsorption isotherm.Biography
Linus N Okoro is lecturing at the American University of Nigeria as an Associate Professor and the chair of petroleum chemistry department. He obtained his PhD in Physical Chemistry from the Dortmund University of Technology, Germany. He has published more than 20 papers in reputed journals.
Email: linus.okoro@aun.edu.ng

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi