Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
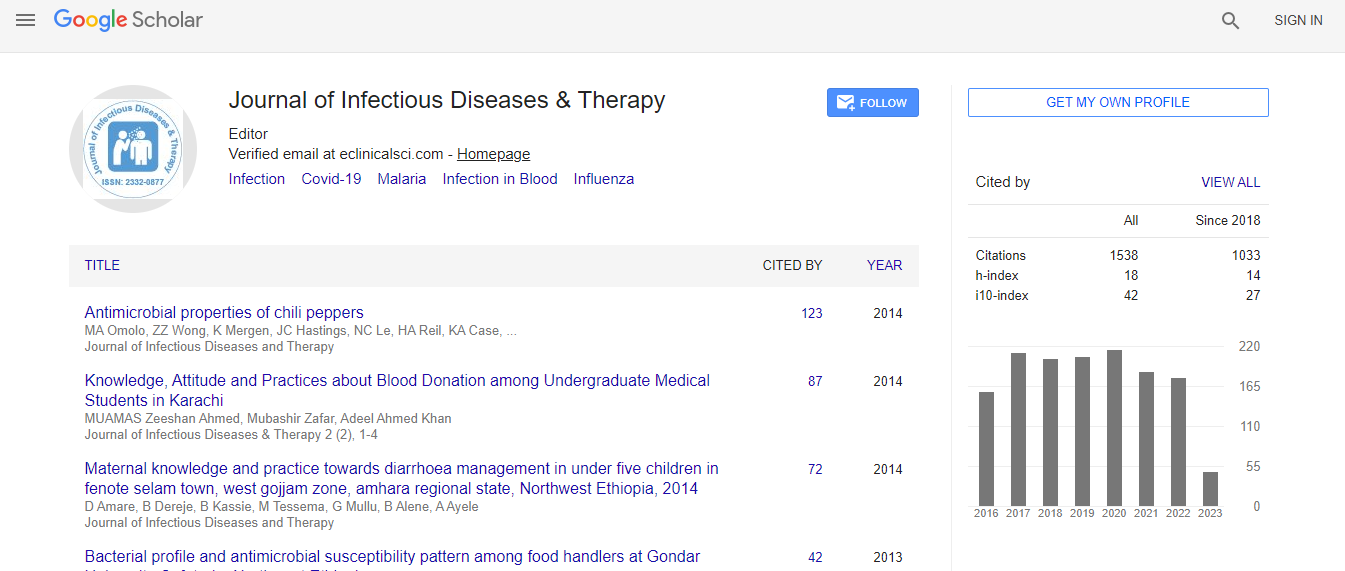
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Contribution of broad spectrum dengue diagnostic technology in the effort to fight dengue global disease burden
Joint Event on 4th Annual Congress on Infectious Diseases & 5th International Conference on Neglected Tropical & Infectious Diseases
Ryen MacDonald
CTK Biotech, USA
ScientificTracks Abstracts: J Infect Dis Ther
Abstract
The increasing burden of dengue virus infection continues to impact populations across the globe, highlighting the importance of multi-parameter dengue diagnostic technologies. Market and literature reviews show that since 2016, there is a soaring demand for early diagnosis of dengue infection, disease discrimination from other flaviviruses such as Zika, and development of accurate serological standards. As a point-of-care IVD company developing and manufacturing dengue antigens, antibodies, and IVD assays for the past decade, CTK Biotech has first-handedly witnessed the extreme limitations in undersourced and remote areas. Thus, as a company, CTK aims to address the issues of quality, ease-of-use, and pricing in an array of dengue products. For early detection of actively replicating virus, CTK offers a real-time PCR test that distinguishes between dengue and related viruses Zika and Chikungunya, and a serotyping assay that identifies dengue types 1-4. For qualification of dengue infection status, they have developed an IgM/IgG antibody test that determines an early or late stage active infection, and an IgG antibody test that identifies past infection only. These products were clinically evaluated in endemic regions, which include Brazil, Venezuela, Colombia, Peru, Mexico, Malaysia, India, and Bangladesh. Because serological standardization and controls for dengue diagnostics remain a challenge, CTK has developed recombinant dengue murine-human chimeric IgM and IgG antibodies, and are currently developing a quantification method using WHO standards for IgG and IgM. As each technology has its advantages, CTK is committed to the fight against dengue infection and aims to contribute from every angle.Biography
Ryen MacDonald completed her PhD in Neurosciences from McGill University and works as a Product Manager for CTK Biotech Inc, Inc located in San Diego, California.
E-mail: rmacdonald@ctkbiotech.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi