Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
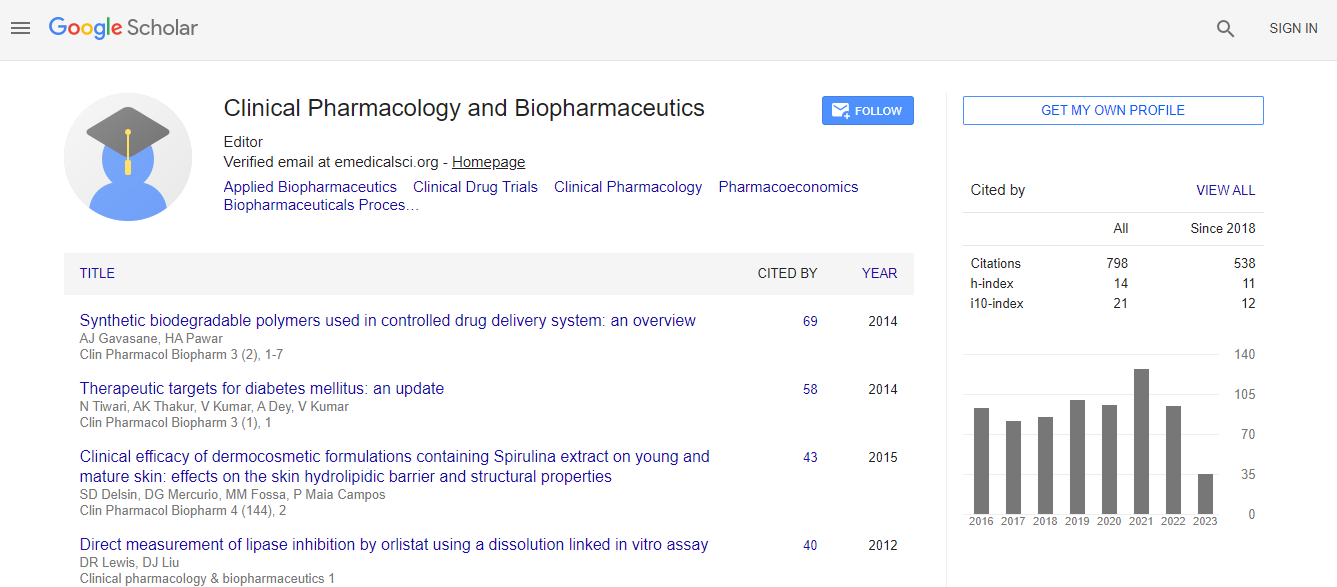
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Clinical development of PfSPZ vaccine: A highly protective, well-tolerated and safe vaccine for the prevention of malaria in travelers and for the elimination of malaria from endemic areas
7th Annual Global Pharma Summit
Thomas Laurence Richie
Sanaria Inc., USA
Keynote: Clin Pharmacol Biopharm
Abstract
Sanaria Inc. has developed methods to manufacture, purify and cryopreserve aseptic Plasmodium falciparum (Pf) sporozoites (SPZ) and is using this platform technology to develop an injectable PfSPZ-based vaccine that provides high-grade, durable protection against infection with Pf malaria. Several candidate vaccines are being developed and tested, including PfSPZ vaccine, in which the PfSPZ are attenuated by irradiation, PfSPZ-CVac, in which fully infectious PfSPZ are attenuated in vivo by concomitant administration of an anti-malarial drug and PfSPZ-GA1, in which the PfSPZ are attenuated by gene knockout. 43 research groups in 15 countries, organized as the International PfSPZ Consortium (I-PfSPZ-C), are collaborating to advance this program by providing intellectual, clinical and financial support. 14 clinical trials of these products have been completed in the USA, Europe and Africa, two are underway and at least 12 more are planned for 2015-2016 in the US (four trials), Germany (2 trials), Tanzania, Kenya, Mali, Burkina Faso, Ghana and Equatorial Guinea. Sanaria anticipates application to license a first generation product as early as late 2017, initially to protect adults and a year later to protect all persons >6 months of age for at least six months. Improved vaccine candidates will be advanced as needed until the following requirements have been met: Long-term protection against natural transmission, excellent safety and tolerability and operational feasibility for population-wide administration. In my presentation, I will describe the three most developed whole PfSPZ vaccine candidates, associated clinical trials, initial plans for licensure and deployment and long-term objectives for a final product suitable for mass administration to achieve regional malaria elimination and eventual global eradication.Biography
Thomas Laurence Richie has earned his PhD and MD degrees from the University of Pennsylvania. He has completed his Residency in Internal Medicine at Massachusetts General Hospital and a Fellowship in Infectious Diseases at Johns Hopkins Hospital. He has directed the US Navy Malaria Program 2004-13, focusing on gene-based malaria vaccines. In 2014, he has joined Sanaria as a Chief Medical Officer where he is responsible for the clinical development of three whole sporozoite-based vaccines. He is currently providing oversight for 12 clinical trials of Sanaria’s products including trials conducted in the USA, Germany, the Netherlands, Mali, Ghana, Burkina Faso, Kenya, Tanzania and Equatorial Guinea.
Email: trichie@sanaria.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi