Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
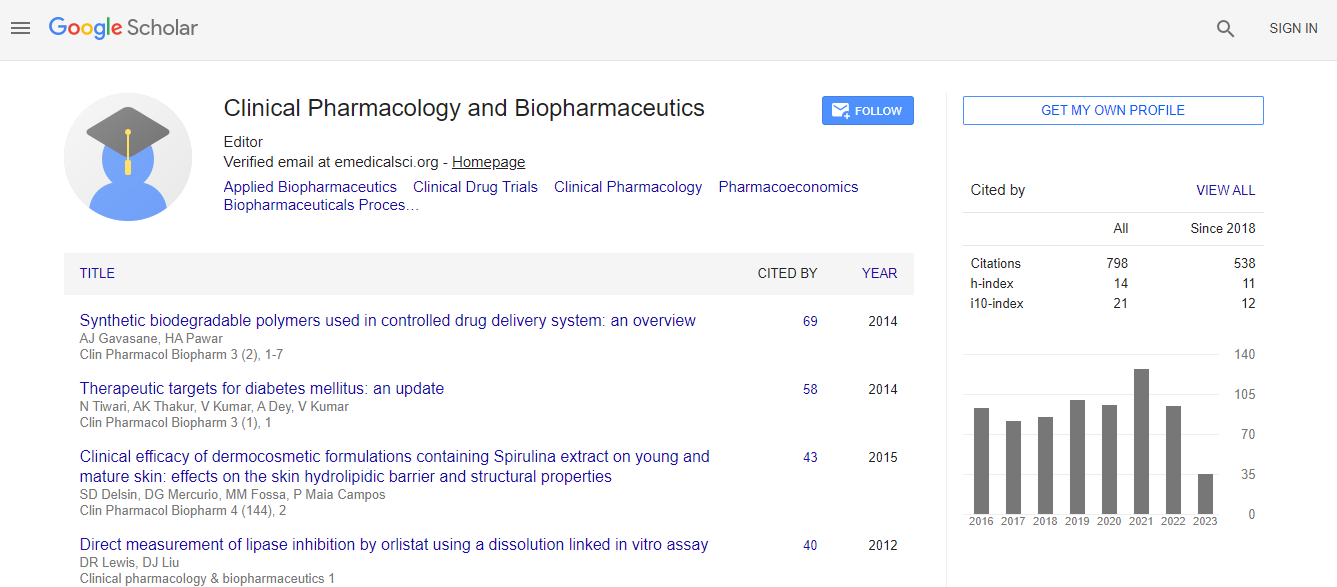
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Characterizing the benefits of pharmacological treatments for promoting the regenerative capacity of cardiac progenitor cells
International Conference and Expo on Biopharmaceutics
Keerat Kaur, Carol A Eisenberg and Leonard M Eisenberg
New York Medical College, USA
ScientificTracks Abstracts: Clin Pharmacol Biopharm
Abstract
Stem cell therapy has been widely used in attempts to repair and regenerate the diseased heart. Since the heart does contain endogenous Cardiac Progenitor Cells (CPCs), heart tissue itself has served as a stem cell source for cardiac repair. Impediments in using CPCs for treating human patients are that the cells are present in low numbers within the heart and require heart biopsies, which may not be efficacious for severely diseased individuals. Two major objectives in optimizing the therapeutic utility of CPCs are to enhance their cardiac regenerative capacity and expand their numbers without sacrificing their cardiac competency. Our laboratory has focused on utilizing pharmacological approaches for promoting the myocardial potential of adult stem cells. Two of the drugs we have investigated are the DNA demethylation reagent 5-azacytidine and the G9a histone methyltransferase inhibitor BIX01294. 5-azacytidine has been widely used for promoting the cardiac differentiation of both adult CPCs and stem cells from non-cardiac tissues, such as the bone marrow. However, 5-azacytidine has long been employed as a tool for stimulating skeletal myogenesis. Since it was unclear whether the ability of 5-azacytidine to promote both cardiac and skeletal myogenesis is dependent strictly on the native potential of the starting cell population or if this drug is a trans-differentiation agent, we examined this drug├ó┬?┬?s effect on cultures of adult mouse atrial tissue, which contains cardiac but not skeletal muscle progenitors. Exposure to 5-azacytidine caused atrial cells to elongate and induced expression of the skeletal myogenic transcription factors MyoD and myogenin. Treatments with 5-azacytidine also allowed atrial cells to undergo skeletal myogenesis when subsequently cultured in differentiation media. The presence of skeletal myocytes in atrial cultures was indicated by dual staining for myogenin and sarcomeric ├?┬▒-actin. Studies from our laboratory have shown that BIX01294 exposure induces precardiac marker expression by bone marrow-derived stem cells and allows these cells to undergo myocardial differentiation when subsequently exposed to cardiogenic stimuli. Here, we report that treating atrial CPCs with BIX01294 enhanced their proliferation without changes in their molecular profile or compromising their cardiac competency. Moreover, fully differentiated cardiomyocytes treated with BIX01294 suffered no changes in their cell phenotype or their contractile activity, which suggests BIX01294, unlike 5-azacytidine, does not possess negative effects that would undermine its use for cardiac repair. Together these data demonstrate that BIX01294 can act as an expansion factor for endogenous CPCs and thus may have utility as an agent that can generate large numbers of native CPCs for treating heart diseases.Biography
Keerat Kaur is currently a PhD student in Department of physiology at New York Medical College, NY. she have completed her undergraduate and master degree in the field Human Genetics from Guru Nanak Dev University, Amritsar, India. Presently, she is working on the project entitled ‘Examining pharmacological approaches for enhancing the cardiac regenerative capacity of adult stem cells’. In the ongoing project, she is trying to characterize the benefits of pharmacological treatments for enhancing the potential of adult tissue derived stem cells to form myocardial tissue. As, stem cell therapy has been widely accepted for its ability to give rise to differentiated cells, we are constantly developing new culture conditions that would allow to enhance the number of stem cells and also differentiate them into mature cardiac tissue. In the second project, she is examining the capability of human bone marrow cells to produce functional cardiomyocytes which can later become a source of fully differentiated cells for transplantation. The long term goal of her study is to focus on procedures that would allow the adult stem cells as a real target for clinical management.
Email: keerat08@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi