Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
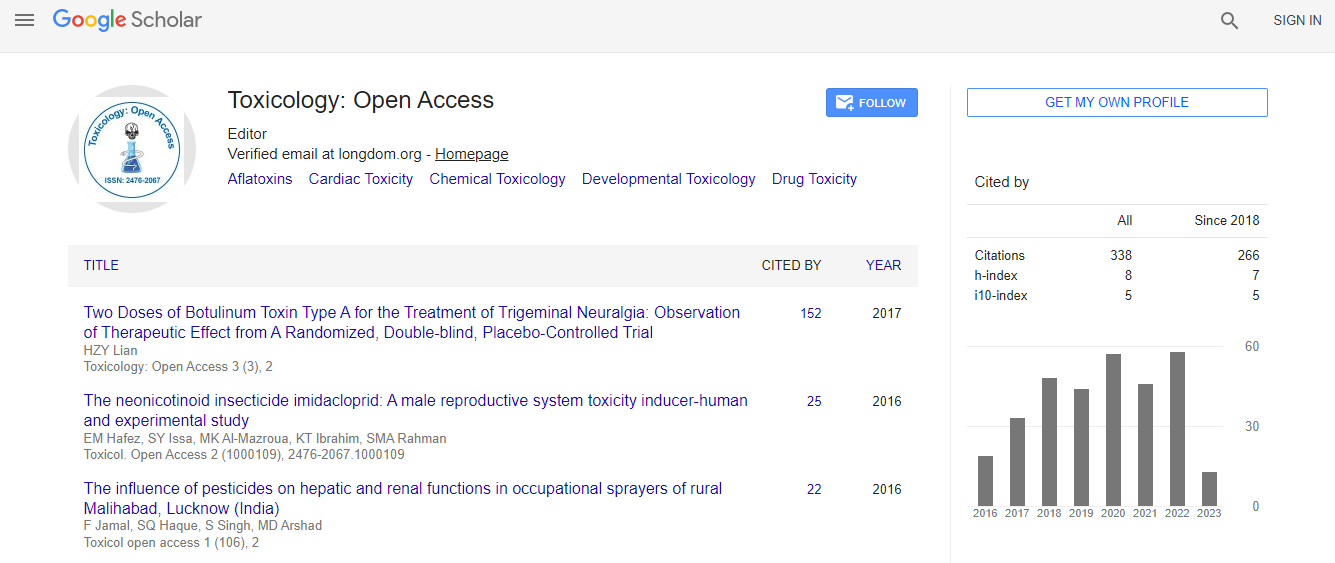
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Bridging the MOA and the POC for clinical application: A case study
14th World Congress on Toxicology and Pharmacology
Hanayuki Okura and Akifumi Matsuyama
National Institute of Biomedical Innovation, Health and Nutrition, Japan
Posters & Accepted Abstracts: Toxicol Open Access
Abstract
Once the manufactured cells were assigned for cell-based medicinal products, non-clinical studies shall be conducted. In the constructed non-clinical study package including in vitro and/or in vivo studies: (1) the mode of action (MOA) should be shown, (2) the proof of concept (POC) to be acquired and (3) safety of the candidates be examined. In this presentation, we will focus on MOA and POC and bridge these two concepts, in which adipose tissue-derived multi-lineage progenitor cells (ADMPCs) would be developed as cell-based medicinal products for liver fibrosis. To treat the patients with the liver cirrhosis, the pathogenesis and pathophysiology should be concerned to the MOA. Liver fibrosis is characterized by excessive accumulation of extracellular matrix with inflammatory status in situ, therefore, in the developing cell-based medicinal products, anti-inflammatory cytokines and fibrinolytic enzyme secretion is anticipated as MOA. After showing the appropriate MOA, the MOA and the POC should be bridges and the key issues are what kind of animal models should be selected. In the case of the developing cell-based products, MOA is that the cells act as vehicle for the delivery of anti-inflammatory cytokines and MMPs. Tetra carbon chloride (CCl4)chronic induction evolved radicals, followed by inflammation, resulted in fibrosis of the parenchyma. So, the expected mode could be applicable in this animal model. To acquire the POC after bridging to MOA, the appropriate route of administration (ROA) should be concerned. In the case study, the POC of the developing cell-based products by the improvement of liver fibrosis, function by systemic administration of ADMPC. In conclusion it can be said that: (1) MOA should be planned from the pathophysiology of the target diseases, (2) the POC-study should be designed to bridge to the MOA and (3) the applicable limitation should be concerned in clinical use for the patients of the disease. Recent Publications 1. Okura H, Morita M, Fujita M, Naba K, Takada N, Takagaki Y, Suzuki O, Matsuyama A (2017) Therapeutic potential of adipose tissue-derived multi-lineage progenitor cell-sheets on liver fibrosis. Journal of Translational Science; 4: 1-6. 2. Negoro T, Okura H, Matsuyama A (2017) Induced Pluripotent Stem Cells: Global Research Trends. Biores Open Access; 6: 63-73.Biography
Hanayuki Okura has completed her PhD degree from Osaka University Graduate School of Medicine. She was Research Fellowship for Young Scientists of Japan Society for the Promotion of Science in her graduate school student years. She is currently the Deputy Director of Center for Rare Diseases Research, National Institutes of Biomedical Innovation, Health and Nutrition, Japan.
Email:haookura-circ@umin.ac.jp

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi