Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
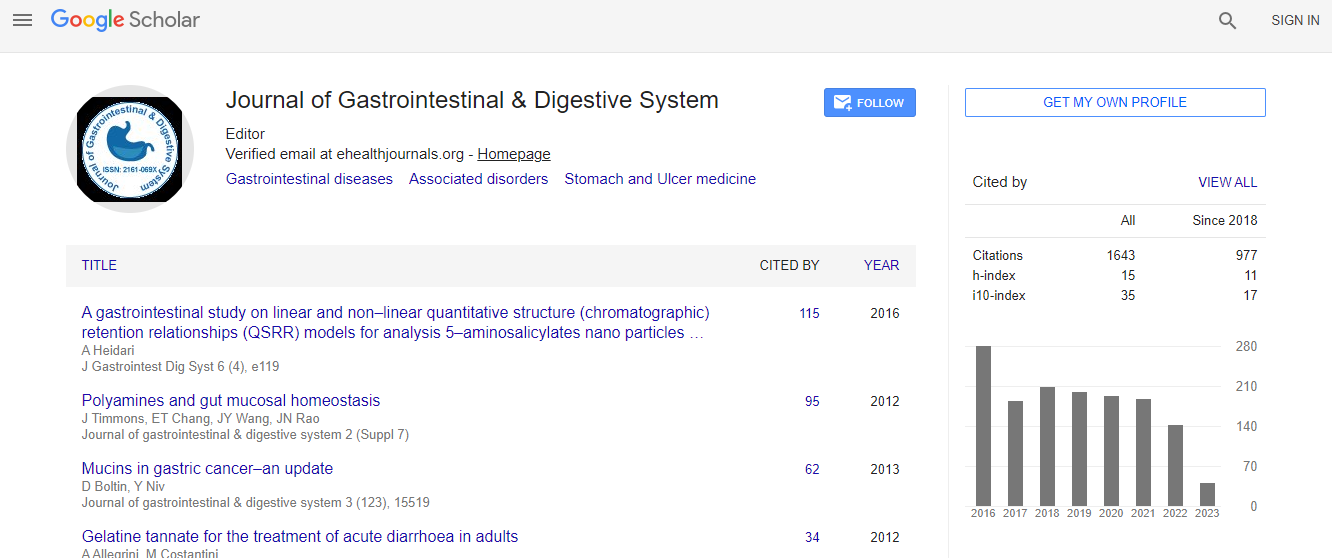
Google Scholar citation report
Citations : 2091
Journal of Gastrointestinal & Digestive System received 2091 citations as per Google Scholar report
Journal of Gastrointestinal & Digestive System peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Bio-similar in inflammatory bowel disease: A review of post-marketing experience
11th World Gastroenterologists Summit
Vito Annese
Valiant Clinic, UAE Careggi University Hospital, Italy
ScientificTracks Abstracts: J Gastrointest Dig Syst
Abstract
The introduction of anti-tumor necrosis factor alpha (anti-TNFa) antibodies about two decades ago has revolutionized the management of inflammatory bowel disease (IBD). However, they are also expensive and their cost can lead to restricted access for many patients. Infliximab has been the first anti-TNF├?┬▒ agent to be used and the first to lose the patent, whereas CT-P13 (Celltrion) has been the first infliximab bio-similar to be available. The clinical trial program of CT-P13 has been performed in patients with ankylosing spondylitis (AS) and rheumatoid arthritis (RA). Both were randomized, double-blind, multinational trials with 30 weeks of treatment, subsequently followed-up to 54 weeks with a further open-label 48-week extension and switch from infliximab to CT-P13. All these studies have demonstrated pharmacokinetic equivalence and clinical efficacy in both AS and RA up to 102 weeks, also after switching. The indication of CT-P13 has been extended since 2013 by EMA and more recently by FDA and Health Canada also to IBD. So far no controlled trials are available in IBD therefore we have made an extensive review of the available open label case series of IBD patients treated across the world. More than 600 IBD patients were evaluated, 39 in pediatric age and 183 switched from infliximab. In most cases, only a clinical evaluation was performed. The mean efficacy was 72%, the rate of adverse events 10.4% and the rate of infusion reactions was 5.5%. These findings are very much comparable to the experience with infliximab, waiting for the data of the controlled trials.Biography
Vito Annese has received his MD in 1981 and subsequently the CCST in Internal Medicine and Gastroenterology at the Catholic University of Rome Italy. He has over 30-years of experience in gastroenterology, with specific interest in functional and inflammatory bowel disorders. He authored more than 250 peer reviewed publications. In the last 10-years he has been head of Gastroenterology at the Research Hospital of S. Giovanni Rotondo and at the University Hospital Careggi of Florence and aggregate professor at the University of Foggia and Florence in Italy. Since September 2016 is Consultant Gastroenterologist at the Valiant Clinic of Dubai.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi