Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
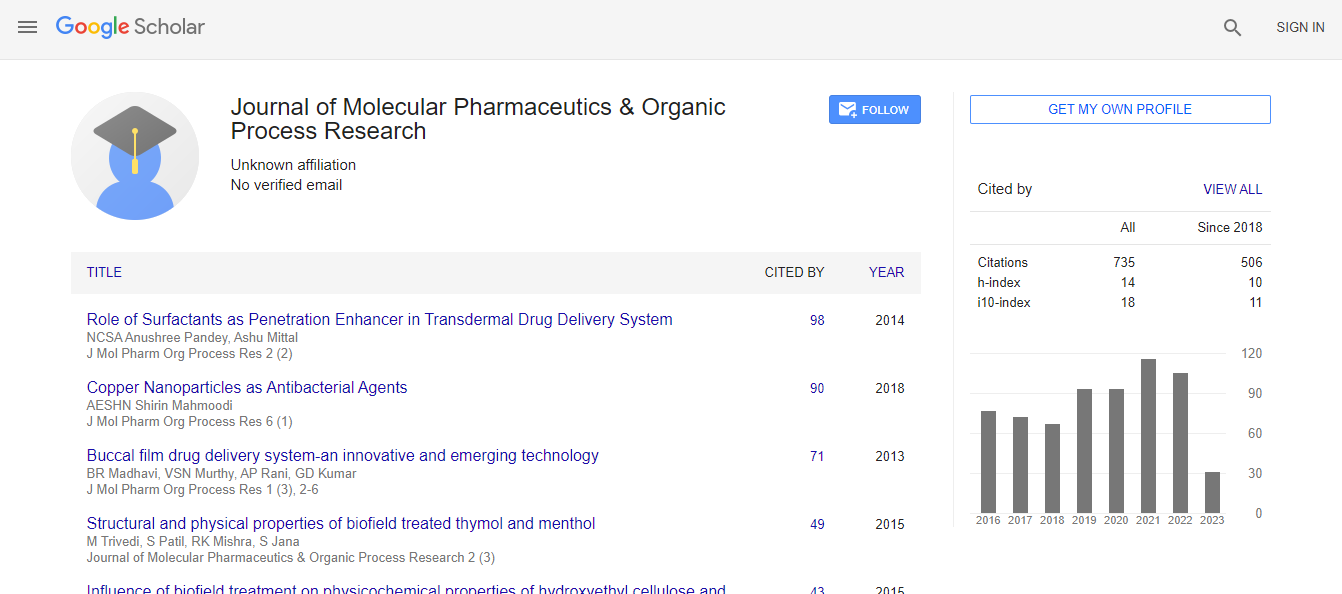
Google Scholar citation report
Citations : 1057
Journal of Molecular Pharmaceutics & Organic Process Research peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Share This Page
Bioavailability enhancement of poorly water-soluble drugs (BCS Class II and IV Drugs) using Hot-Melt Extrusion (HME): The cost-effective approach
2nd International Conference on Pharmaceutical Formulations and API
Devendra Ridhurkar
Neurax Pharmaceuticals, Spain
ScientificTracks Abstracts: J Mol Parm Org Process Res
Abstract
For orally administered drugs, solubility and permeability is one of the rate-limiting factors to achieve their desired concentration in systemic circulation for pharma√?¬?√?¬¨cological response. Poor solubility of BCS class II and IV drugs is attributable for delay or failure and due to this reason formulation scientist faces a major challenge to develop a formulation with good bioavailability. Enhancement of solubility and bioavailability of poorly soluble drugs can be achieved by amorphous solid dispersions which are prepared by converting the poorly water-soluble crystalline form into a more soluble amorphous form within the polymeric blends. Hot Melt Extrusion (HME) has been widely used to prepare amorphous solid dispersions for the improvement of solubility and dissolution rates of poorly soluble materials. During the melt extrusion process, the dissolution of APIs into the polymer matrix is accelerated under the influence of shear and heat. HME has gained popularity in the pharmaceutical industry as a means of improv√?¬?√?¬¨ing the bioavailability of drugs due to its wide applications, simple pro√?¬?√?¬¨cess and low costs. HME is an efficient technology for producing solid molecular dispersions with considerable advantages including the absence of solvents, few processing steps, and con√?¬?√?¬¨tinuous operation over solvent-based processes such as spray drying and co-precipitation. Also, HME is one of the recommended processes by FDA to encourage move from batch-to-continuous manufacturing. Moreover, it can be used to earn intellectual property and to make the noninfringing strategies for products development with ANDA para IV fillings. Marketed formulations Kaletra√?¬?√?¬ģ and Onmel√?¬?√?¬ģ which are prepared by HME technology are the classical examples.Biography
Ridhurkar works as an Expert Scientist at Neurax Pharm., Barcelona. He is Subject Matter Expert over 16 years of scientific leadership and management experience in development and manufacturing of NCE, Proprietary and Generic (Complex, Specialty and Branded) for global pharma majors like Servier, Hungary, Dr. Reddys India. He is expert in using platform technologies like hot melt extrusion, nanotechnology, and cyclodextrin complexation. He obtained his M. Pharm, PhD degree in Pharmaceutics from IIT, Varanasi, India. He is a member of editorial board for various pharmaceutical journals and has earned to his credit over 10 peer-reviewed papers in reputed international and national journals and 9 patents to his credit. He has been associated with various pharmaceutical bodies in Hungary, India and American Association of Pharmaceutical Scientists. He is a member of programme advisory committee for Pharma Connect Congress, Hungary and has attended and delivered seminars and presentations at various national and international conferences.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi