Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
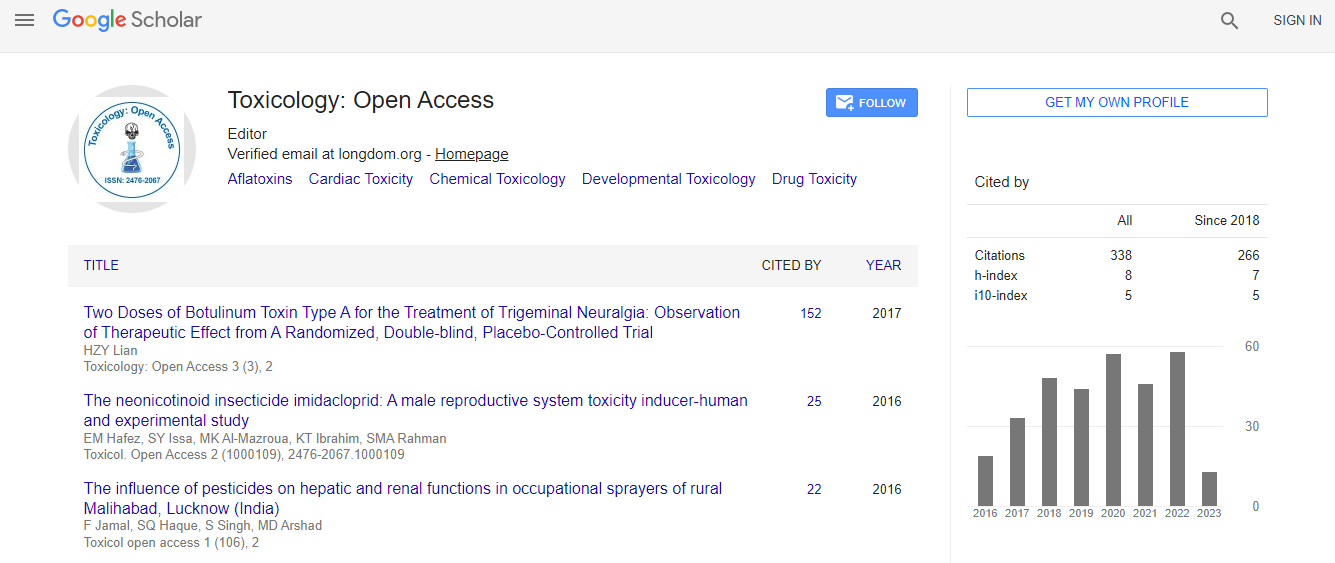
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Arsenic trioxide induces structural perturbation of hen egg white lysozyme towards oligomers formation
20th World Congress on Toxicology and Pharmacology
Satish Kumar and Neelakant Varma
Gujarat Forensic Sciences University, India
Posters & Accepted Abstracts: Toxicol Open Access
Abstract
Arsenic trioxide is one of the most common metallic pollutants entering the food chain both by human activities and nature. Its introduction to living organism and accumulation is known to manifest several metabolic and hormonal disorders; however its role in protein misfolding and aggregation followed by neurodegenerative disorders is not fully elucidated. In the present study by employing several biophysical techniques, we reveal the aggregation mechanism of Hen Egg White Lysozyme (HEWL) in presence of Arsenic Trioxide (As2O3) at physiological condition and characterized the aggregates. Our ThT fluorescence and scattering data shows that As2O3 promote the in vitro aggregation of HEWL in concentration dependent manner. Early phase of aggregation was observed to be induced by exposure of hydrophobic surfaces which later reorganized to promote further self-association leading to β sheet structure which was evident by CD spectroscopy. Presence of lower ordered oligomers after two days and higher ordered oligomers along with amorphous aggregates, as evident by AFM after week long incubation, indicate that As2O3 drives the self-assembly of lysozyme towards oligomers form. It is now been believed that not the mature fibrils but the transiently formed oligomers are the real culprit of several neurodegenerative disorders. Though we did not observed any mature fibrils in present study, presence of oligomers of Rh ~62 nm and ~222 nm indicate that heavy metal promotes small and medium sized oligomers which could be potential toxic species of arsenic mediated toxicity. With the fact that several environmental pollutants including heavy metals are continually entering the living organism resulting into various chronic disorders, present study provides a new insight about arsenic driven protein aggregation and toxicity associated with that.Biography
E-mail: satish.kumar@gfsu.edu.in

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi