Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
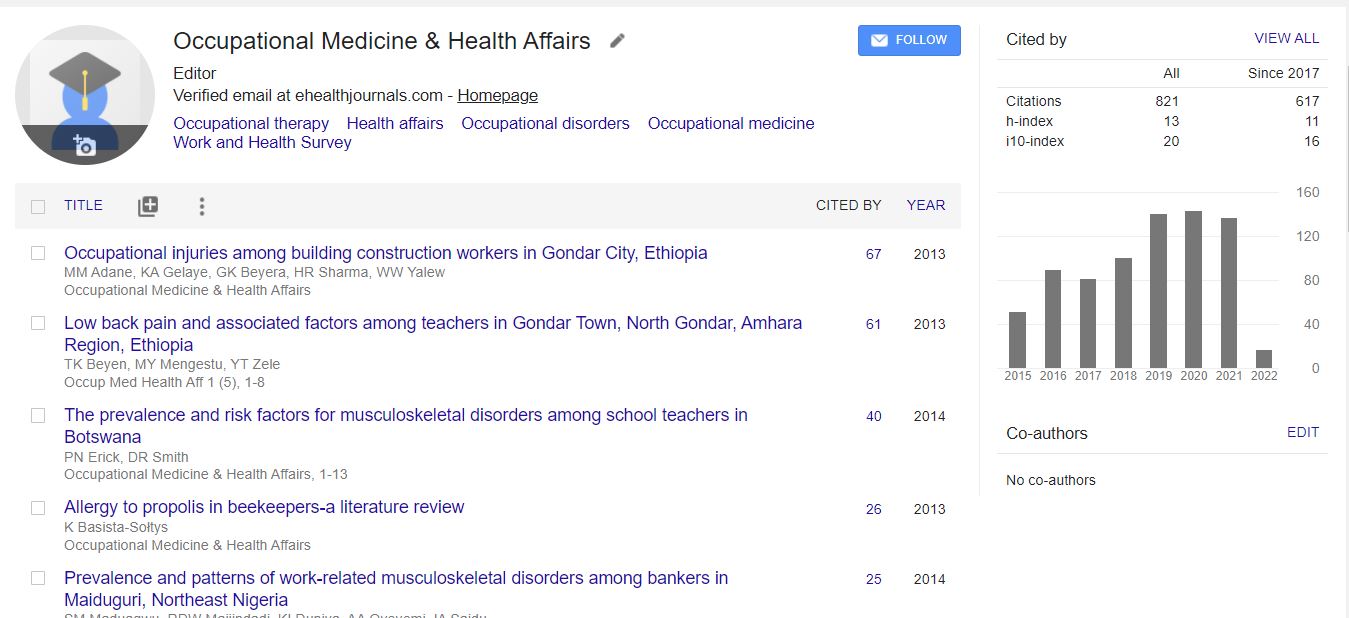
Google Scholar citation report
Citations : 1907
Occupational Medicine & Health Affairs received 1907 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Academic Keys
- China National Knowledge Infrastructure (CNKI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
AMOXICILLIN ADSORPTION ON MICROWAVE PREPARED ACTIVATED CARBON FROM ARUNDO DONAX LINN: ISOTHERMS, KINETICS, AND THERMODYNAMICS STUDIES
International Conference on Environmental Health & Safety
Muthanna J. Ahmed
University of Baghdad, Iraq Universiti Kebangsaan Malaysia, Malaysia
Posters & Accepted Abstracts: Occup Med Health Aff
Abstract
Microwave- assisted KOH activation of renewable biomass Arundo donax Linn was adopted for preparation of activated carbon (KAC) with high capacity for amoxicillin antibiotic (AMX). The characteristics of KAC were examined by proximate and pore structure analyses, scanning electron microscopy (SEM) and Fourier transforms infrared spectroscopy (FTIR). The ash and moisture contents of KAC were 5.5 and 0.5 % compared to 2.2 and 2.1 % for raw biomass. The BET surface area and total pore volume were identified to be 1065.3 m�²/g and 0.643 cm�³/g, respectively. The best preparation conditions in terms of KAC yield and AMX uptake were reported as 10 min radiation time, 620 W radiation power and 2 g/g impregnation ratio resulted in 9.1 % yield and 196.9 mg/g AMX uptake. Experimental equilibrium data for AMX adsorption were analyzed by Langmuir, Freundlich, and Sips isotherms. The results showed that the best fitting was achieved by Sips isotherm with high adsorption capacity of 345.4 mg/g on KAC compared to 75.8 mg/g on precursor. Also, kinetic data were correlated by pseudo-first order, pseudo-second order, and intraparticle diffusion models with well-fitting to pseudo-second order model. Thermodynamic analysis showed that adsorption enthalpy of AMX was 17.7 kJ/mol which revealed endothermic and physisorptive nature under examined conditions.Biography
Email: muthanna.ja@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi