Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
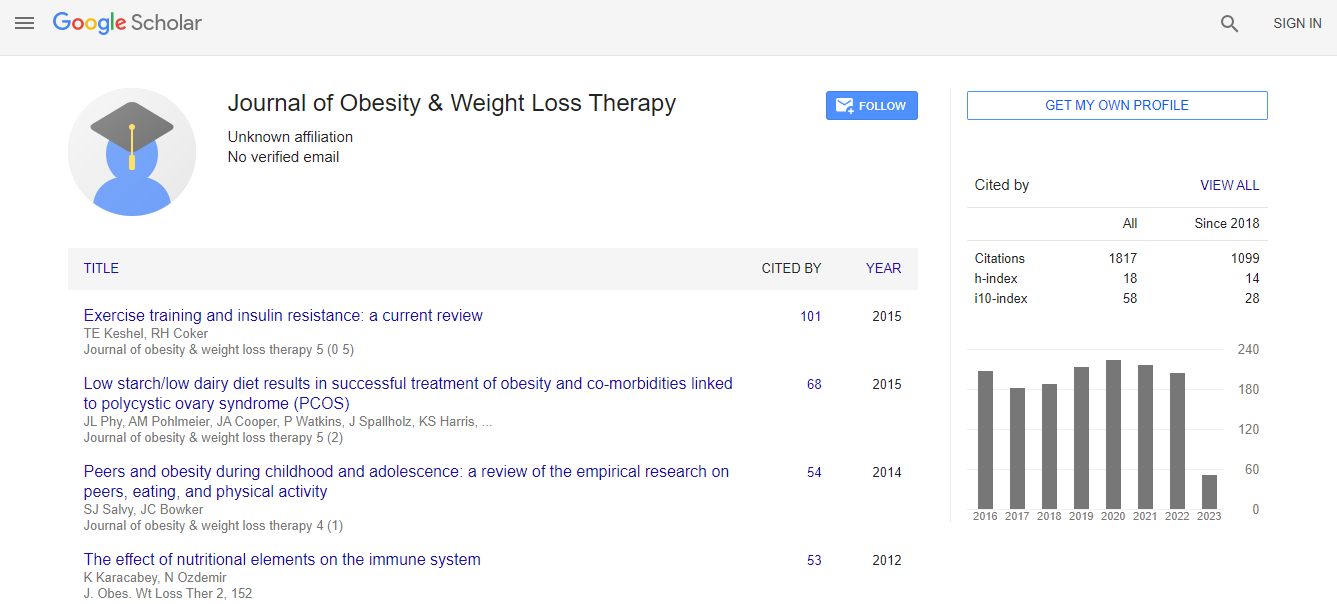
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Accuracy of the Arkray glucocard 01 as it relates to the ISO 15197: 2013 requirements for blood glucose meter systems for self- monitoring of diabetes mellitus
3rd International Conference and Exhibition on Obesity & Weight Management
John Gleisner, Patricia Gill and Julie Walker
ARKRAY, USA
Posters: J Obes Weight Loss Ther
Abstract
Background: Blood Glucose Monitoring Systems (BGMS) are an important tool in the management of diabetes. The gold standard in measuring the accuracy of BGMS in the self-testing of diabetes mellitus is known as the ISO 15197:2013. The level of accuracy of the BGMS results in a personâ�?�?s ability to regulate their blood sugar levels. According to ISO 15197:2013, system accuracy performance criteria is defined as 95% of the BGMS results falling within �?±15 mg/dL of the reference analyzer results with glucose concentrations less than 100 mg/dL. For samples with glucose concentrations â�?¥100 mg/dL, 95% of the BGMS results need to be within 15% of the reference analyzer results. Furthermore 99% of all results are required to be in the A and B zones of the Consensus Error Grid. Purpose: The objective of this study is to demonstrate whether the ARKRAY GLUCOCARD 01 aligns with the newly introduced BGMS self-monitoring performance requirements. Methods: Two lots of ARKRAY GLUCOCARD 01 blood glucose test strips were evaluated for performance and bias comparison (N=120 data points). The samples were collected from the fingertip of confirmed diabetics by trained personnel in September 2014 at the ARKRAY Factory, Inc. Reference values were obtained using the YSI Model 2300 Analyzer. The data was analyzed using the minimum system accuracy performance criteria published in the ISO 15197:2013. Results: The results showed that 100% of the <100 mg/dL samples (n=8/8) were �?± 15 mg/dL thus exceeding the 95% accuracy criteria. 97.3% of the â�?¥100 mg/dL samples (n=109/112) fell within the pre- determined 15%, which surpassed the 95% performance criteria. All data were within the A and B zones of the Consensus Error Grid. The overall bias was -0.1% demonstrating a strong agreement between the GLUCOCARD 01 and the YSI reference analyzer, which is considered the gold standard glucose assay for BGMS studies. The correlation coefficient (r) =0.98 demonstrates a strong linear relationship between the YSI and meter result. Conclusion: The data acquired on the ARKRAY GLUCOCARD 01 met the ISO 15197:2013 system accuracy performance criteria, the most stringent BGMS requirement in the self-monitoring of diabetes mellitus.Biography
John Gleisner completed his PhD in Biochemistry from the University of Minnesota and postdoctoral studies from the University of Iowa. His first career following graduate school was at the Virginia Mason Research Center in Seattle, WA. He later moved into industry where he is currently the Science Director at ARKRAY Factory USA in Minneapolis, MN. He has spent nearly 30 years working on blood glucose system development and support. He has authored over 25 publications and holds 11 US patents.
Email: walkerj@arkrayusa.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi