Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
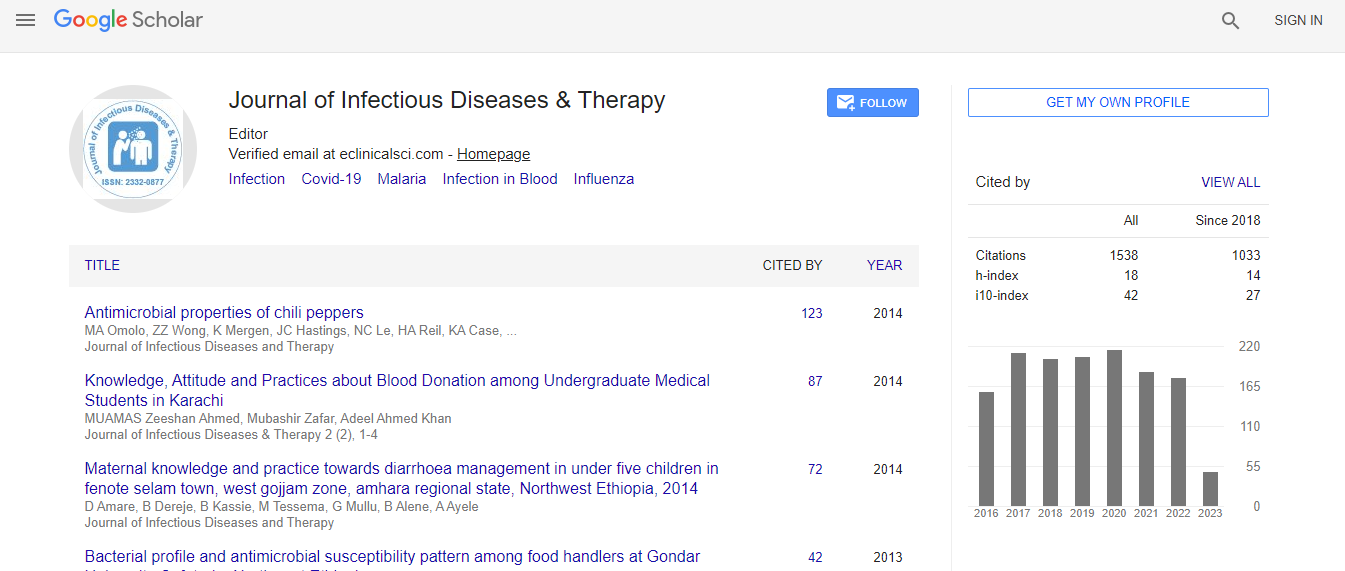
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
A comparison of short course combinations with AmBisome for the treatment of visceral leishmaniasis (VL) in Bangladesh
3rd Euro-Global Conference on Infectious Diseases
Md Ridwanur Rahman, Kazi M Jamil, M A Faiz, Rashedul Haque and Rasheda Samad
Shaheed Suhrawardy Medical College, Bangladesh International Centre for Diarrhoeal Disease Research, Bangladesh
ScientificTracks Abstracts: J Infect Dis Ther
Abstract
AmBisome therapy for VL has an excellent efficacy and safety profile and has been adopted as a first line regimen in Bangladesh. Second line treatment options are limited and should preferably be given in short course combinations in order to prevent the development of resistant strains. Combination regimen including AmBisome, paromomycin and miltefosine proved to be safe and effective in the treatment of Indian VL. In the present study, the safety and efficacy of these same combinations were assessed in field conditions in Bangladesh. The safety and efficacy of three combination regimens: A 5 mg/kg single dose of AmBisome+7 subsequent days of miltefosine (2.5 mg/kg/day), a 5 mg/kg single dose of AmBisome+10 subsequent days of paromomycin (15 mg/kg/day) and 10 days of paromomycin (15 mg/kg/day)+miltefosine (2.5 mg/kg/day) were compared with a standard regimen of AmBisome 15 mg/ kg given in 5 mg/kg doses on day 1, 3 and 5 (at the time of the study the regimen adopted in the National Treatment Guidelines). This was a phase III open label, individually randomized clinical trial, conducted between July 6, 2010 and March 22, 2014. Patients from 5 to 60 year with uncomplicated primary VL were recruited from the Community Based Medical College Bangladesh (CBMC,B) and the Upazila Health Complexes of Trishal, Bhaluka and Fulbaria (all located in Mymensingh district) and randomly assigned to any of the treatments. The objective was to assess definite cure at 6 months after treatment. Randomization sequences were generated centrally and stratified per site with allocation concealment by opaque and sequentially numbered sealed envelopes. 601 patients recruited between July 2010 and March 2014 received either AmBisome monotherapy (n=158), AmBisome+paromomycin (n=159), AmBisome+miltefosine (n=142) or paromomycin+miltefosine (n=142). At 6 months after treatment, final cure rates for the intentionto- treat population were 98.1% (CI 96, 0-100) for AmBisome monotherapy, 99.4% (CI 98.2-100) for the AmBisome+paromomycin arm, 94.4% (CI 90.6-98.2) for the AmBisome+miltefosine arm and 97.9% (CI 95.5-100) for paromomycin+miltefosine. The perprotocol population was 587. Three patients died and there were no relapses and PKDL. All treatments were well tolerated with no unexpected side effects. Adverse events were most frequent during treatment with miltefosine+paromomycin and three major safety events occurred in this arm, which all resolved after treatment was stopped. None of the combinations was inferior to AmBisome in both the intention-to-treat and per-protocol populations. All the combinations demonstrated excellent overall efficacy, were well tolerated and safe and could be employed under field conditions in Bangladesh.Biography
Md Ridwanur Rahman is currently working as a Professor of Internal Medicine in a Public Medical College at Dhaka, Bangladesh since 2006. Beside teaching and training of undergraduates & postgraduates, he also works as a Consultant Internist in a public tertiary care hospital and as a Researcher on Neglected Tropical & Infectious Diseases including snake-bite and poisoning. He has published more than 40 articles in different journals.
Email: ridwanurr@yahoo.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi