
Page 65
conferenceseries
.com
Volume 8
Gynecology & Obstetrics
ISSN: 2161-0932
Gynecology 2018
October 08-10, 2018
October 08-10, 2018 | Zurich, Switzerland
5
th
International Conference on
Gynecology and Obstetrics
Development and application of nanotechnology based multiplexed microELISA system for remote
antenatal populations
Michael J Sinosich
1
, B Govind
2
, A Abhyankar
2
, B Rushton
2
, N Chau
2
, R Sidorov
2
, E Ke
2
and
H Moore
2
1
Douglass Hanly Moir Pathology, Australia
2
Pictor Ltd., New Zealand
D
ata is presented on a multiplexed microELISA platform which consists of a PictArray™, plastic slide of 16 microwells.
Each microwell contains 25 defined specific immunoreaction immunoblots, consisting of assay controls and duplicate
tests. Immunoblot intensity is determined by array reader, PictImager, and quantification performed by proprietary software
(Pictorial©). This entire immunoanalysis solution is platform agnostic. Hence, due to its small footprint, superior affordability
and simple technology, testing can be conducted in community health centres or outreach mobile health clinics. Current test
menu includes screening for IgG and IgM for ToRCH and Hepatitis A & E antigens. Whereas, the ENA panel screen for IgG
autoantibodies against nine connective tissue antigens. Testing may be performed on finger prick or with 10
μ
L of serum. Due
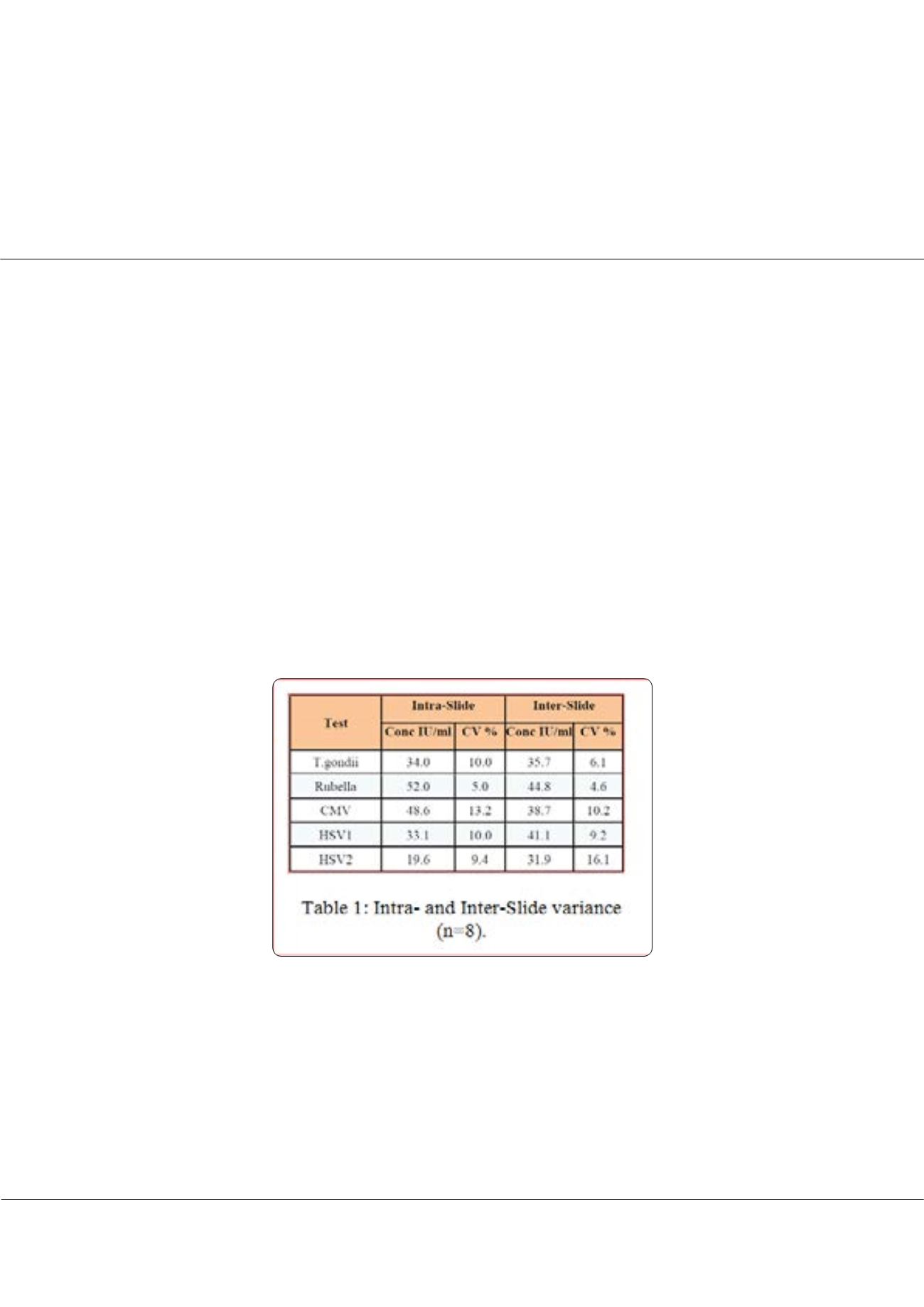
to simple technology, other region specific panels under development include sepsis and fever panels. Array precision profile
compared favourably against established single analyte ELISA platforms and exceeded performance of many immunological
Point-of-Care products. Direct comparison of Pictor Rubella IgG assay against commercial product, showed a significant
correlation (r2) of 0.73. We have developed a versatile and robust multiplexed micro-ELISA test platform. Being platform
agnostic and economical, the PictArray testing platform has been optimised for application to screen all populations, especially
those rural communities which are currently beyond the reach of mainstream healthcare.
Biography
Michael J Sinosich has completed his PhD on Trophoblast Physiology and PAPP-A. His research interests include non-invasive assessment of fetomaternal
wellbeing. He is the Director of Prenatal Testing (DHM Pathology) and serves as Consultant at Pictor Ltd, a developer and manufacturer of multiplexed microELISA
assay platform. He has published/presented numerous papers in reputed journals and holds several patents.
msinosich@sonichealthcare.com.auMichael J Sinosich et al., Gynecol Obstet 2018, Volume 8
DOI: 10.4172/2161-0932-C4-033
















