Research Article Open Access
Withdrawal from Buprenorphine/Naloxone and Maintenance with a Natural Dopaminergic Agonist: A Cautionary Note
Kenneth Blum1,4-10*, Marlene Oscar-Berman2, John Femino3, Roger L Waite4, Lisa Benya5, John Giordano6, Joan Borsten5, William B Downs4, Eric R Braverman7, Raquel Loehmann7, Kristina Dushaj7, David Han8, Thomas Simpatico9, Mary Hauser10, Debmalya Barh11 and Thomas McLaughlin121Department of Psychiatry and McKnight Brain Institute, University of Florida, College of Medicine, Gainesville, Florida, USA
2Boston VA and Boston University School of Medicine, Boston Massachusetts, USA
3Meadows Edge Recovery Center, North Kingstown Rhode Island, USA
4Department of Nutrigenomics, LifeGen, Inc. Austin, Texas, USA
5Department of Clinical Medicine, Malibu Beach Recovery Center, Malibu Beach, California, USA
6Department of Holistic Medicine, G & G Health Care Services, LLC., North Miami Beach, Florida, USA
7Department of clinical Neurology, PATH Foundation NY, New York, USA
8Department of Management Science and Statistics, the University of Texas at San Antonio, San Antonio, Texas, USA
9Community Mental Health Institute, Center for Clinical & Translational Science, University of Vermont and Department of Psychiatry, University of Vermont College of Medicine, Burlington, Vermont, USA
10Dominion Diagnostics, Inc. North Kingstown, Rhode Island, USA
11Center for Genomics and Applied Gene Technology, Institute of Integrative Omics and Applied Biotechnology (IIOAB), Nonakuri, Purbe Medinpur, West Bengal, India
12Center for Psychiatric Medicine, North Andover, Massachusetts, USA
- *Corresponding Author:
- Kenneth Blum, PhD
Department of Psychiatry and McKnight Brain Institute
University of Florida
College of Medicine
PO Box 103424 Gainesville
Florida, USA, 32610-3424
Tel: 619-890-2167
Fax: 352-392-9887
E-mail: drd2gene@gmail.com
Received March 08, 2013; Accepted April 17, 2013; Published April 23, 2013
Citation: Blum K, Oscar-Berman M, Femino J, Waite RL, Benya L, et al. (2013) Withdrawal from Buprenorphine/Naloxone and Maintenance with a Natural Dopaminergic Agonist: A Cautionary Note. J Addict Res Ther 4:146. doi:10.4172/2155-6105.1000146
Copyright: © 2013 Blum K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: While numerous studies support the efficacy of methadone and buprenorphine for the stabilization and maintenance of opioid dependence, clinically significant opioid withdrawal symptoms occur upon tapering and cessation of dosage.
Methods: We present a case study of a 35 year old Caucasian female (Krissie) who was prescribed increasing dosages of prescription opioids after carpel tunnel surgery secondary to chronic pain from reflex sympathetic dystrophy and fibromyalgia. Over the next 5 years, daily dosage requirements increased to over 80 mg of Methadone and 300 ug/hr Fentanyl transdermal patches, along with combinations of 12-14 1600 mcg Actig lollipop and oral 100 mg Morphine and 30 mg oxycodone 1-2 tabs q4-6hr PRN for breakthrough pain. Total monthly prescription costs including supplemental benzodiazepines, hypnotics and stimulants exceeded $50,000. The patient was subsequently transferred to Suboxone® in 2008, and the dosage was gradually tapered until her admission for inpatient detoxification with KB220Z a natural dopaminergic agonist. We carefully documented her withdrawal symptoms when she precipitously stopped taking buprenorphine/naloxone and during follow-up while taking KB220Z daily. We also genotyped the patient using a reward gene panel including (9 genes 18 alleles): DRD 2,3,4; MOA-A; COMT; DAT1; 5HTTLLR; OPRM1; and GABRA3.
Findings: At 432 days post Suboxone® withdrawal the patient is being maintained on KB220Z, has been urine tested and is opioid free. Genotyping data revealed a moderate genetic risk for addiction showing a hypodopaminergic trait. This preliminary case data suggest that the daily use of KB220Z could provide a cost effective alternative substitution adjunctive modality for Suboxone®. We encourage double-blind randomized –placebo controlled studies to test the proposition that KB220Z may act as a putative natural opioid substitution maintenance adjunct.
Keywords
Buprenorphine/naloxone; Withdrawal; Natural dopaminergic agonist
Introduction
Currently, the most effective treatment for the growing prescription opioid epidemic [1] is opioid replacement therapy (ORT). Each approved medication for ORT has unique characteristics that determine its suitability for an individual patient [2]. Methadone, a synthetic opiate agonist is demonstrably effective in reducing withdrawal symptoms and lowering the rate of IV drug use [3,4]. Buprenorphine derived from Thebaine, is a semi-synthetic opioid, a naturally occurring alkaloid of the opium poppy, Papaver somniferum. Buprenorphine has unique receptor binding characteristics at the mu and kappa opiate receptors that provide partial agonist effects and opioid blockade. Medication assisted therapy with Methadone and buprenorphine provide significant cost benefit and cost efficiencies, especially when compared to the costs of medical, social and legal consequences of untreated addiction [5,6]. The desire to find a substance, that has agonistic activity and also blocks opiate-type receptors (mu, delta, etc.), provided the impetus for the development of a combination mu receptor agonist and narcotic antagonist [7]. The history of the use of buprenorphine/naloxone combination Suboxone® has been adequately reviewed elsewhere [8-13] and pertinent information documenting the increase in its use can be obtained from the Federal Center for Substance Abuse Treatment (CSAT) Locator List [10]. Approval of buprenorphine containing medications for the treatment of opioid dependence has doubled since it was first introduced in the US, in 2003. It has been well received in England [14] and Europe as an expansion of treatment options.
Pharmacokinetics of Mixed Agonist/Antagonists of Opiate Receptors
Buprenorphine is morphine-like but is 25 to 50 times more potent [15,16]. It is noteworthy that buprenorphine has very low oral bioavailability because it undergoes extensive first-pass metabolism. However, its bioavailability is substantial enough that sublingual (SL) administration makes this a feasible treatment for opioid dependence. The mean time to peak plasma concentration following SL administration varies and can range from 40 minutes to 3.5 hours. Buprenorphine is highly protein bound (96%) and has a large volume of distribution. It is metabolized extensively to norbuprenorphine by N-dealkylation, primarily through cytochrome P450 (CYP) 3A4. The half-life of buprenorphine is long, and there is considerable variation in reported values of terminal elimination, with mean values ranging from 3 to 44 hours. Most of a dose of buprenorphine is eliminated in the feces, with approximately 10-30% excreted in urine.
Buprenorphine has an extremely high affinity and slow dissociation from opioid receptors, providing simultaneous opioid blockade and agonist effects. The duration of action of buprenorphine is longer than these pharmacokinetic parameters, resulting in suppression of opioid withdrawal symptoms for 2 to 3 days after cessation of use.
Naloxone is added to buprenorphine for the treatment of opiate dependence and to prevent intravenous diversion [11]. However, the efficacy of buprenorphine is not affected by the combination because the half-life for buprenorphine is much longer than for naloxone (up to 32 vs. 1 hour for naloxone) and naloxone is poorly absorbed sublingually relative to buprenorphine. Plasma levels of naloxone are lower and decline much more rapidly than those for buprenorphine [17-19]. The pharmacokinetics of buprenorphine does not appear to be influence by the presence of naloxone [17-26]. However, the presence of naloxone does appear to reduce the degree of appeal and reward, supporting the recommendation for combination products over mono products for longer term prescribing for high risk individuals [13].
Toxicity from methadone and the risks of a drug overdose and death is much higher than for buprenorphine. Methadone has complex metabolism that is quite variable secondary to genetic polymorphisms and multiple metabolic pathways. Phase 1 metabolism of nearly 80% of pharmaceutical drugs in use today is the province of enzymes that are synthesized by the cytochrome P450 (CYP) family. Gene duplications and gene polymorphisms CYP2D6, CYP2C19 and CYP2C9 are most frequently responsible for variations in drug metabolism. Approximately 5% of Europeans, 1% of Asians, 5-14% of Caucasians and 5% of Africans lack CYP2D6 activity. These individuals are known as poor metabolizers [24,25]. Although methadone and buprenorphine are metabolized by the 3A4 system, methadone metabolism also involves 2D6 and 2B6, resulting in a significant risk for clinically significant drug interactions with methadone compared to buprenorphine. Enzyme inhibitors and inducers of the 3A4 system can change levels of methadone and buprenorphine, however, the partial agonist effects of buprenorphine appear to protect against overdose mortality. 3A4 inducers such as carbamazepine and barbiturates result in clinically significant lowering of methadone drug levels, resulting in the emergence of withdrawal symptoms and raising of methadone doses for symptom stabilization. Notably, Seifert [27] found better clinical outcomes and more effective short-term relief of affective disturbances resulted from combining buprenorphine and carbamazepine than from combining carbamazepine with methadone when detoxifying multi-drug opiate addicts. An explanation of this interesting effect may be that while buprenorphine has similar effects on enzyme induction as methadone the reported difference could be due to buprenorphine’ high affinity and slow dissociation receptor binding characteristics.
Potential Anti-reward Properties of Long Term Opioid Replacement Therapy (ORT)
Blum et al. [13] have adequately reviewed the potential anti-reward mechanisms associated with the buprenorphine/naloxone combination; here once again we turn our attention to the potential anti-reward properties of long term ORT.
Opioid withdrawal symptoms resolve with the reinstitution of agonist medications and thereby an effective reduction in relapse that occurs early in the treatment process. Cravings associated with withdrawal symptoms resolve with stabilization of the opioid system, as evidenced by much higher rates of relapse or inability to complete opioid detoxification when non-cross tolerant medications, such as Clonidine, are used. However, long term, relapse rarely results from the appearance of withdrawal symptoms, but is secondary to cravings that arise from cue reactivity, persistent anhedonia and reduced stress responsiveness.
Given that naloxone, blocks the effects of all opiates, it has for many recovering addicts facilitated the transformation to a drug-free state and it also reduced the detoxification period. Theoretically, the use of naloxone or naltrexone alone at therapeutic doses also blocks the release of DA in the reward site of the brain [13,28,29]. While, clinically many patients on naltrexone alone may still experience pleasure and may not always experience anhedonia, this suggests a highly complex mechanism.
Experiments [30] have shown that mu-receptor binding of agonist like morphine or potentially heroin, are significantly reduced with buprenorphine. Although the outcome for short-term therapy is beneficial, in the long term, administration of buprenorphine will result in an attenuation of mu receptor occupancy of the natural opioid enkephalin and will eventually, like naloxone, result in reduced release of DA at the accumbens brain region [31-34]. This, in turn, may lead to generalized drug seeking behavior. Moreover, several cases of acute hepatitis and fatal overdose have been reported with buprenorphine [35-41].
An important question to answer is “What effect does the long-term administration of buprenorphine/naloxone have, on dopaminergic reward pathways?” It has been assumed that opiate receptors, mostly on mu receptors, are the sole site of Morphine activity. Several studies have demonstrated that dopaminergic agonists and antagonists and opiate mu agonists at exceptionally high concentrations are incapable of binding to each other’s receptors. Other neurotransmitters are also unable to bind to opiate receptors they include; acetylcholine, norepinephrine, serotonin, gamma-hydroxybutyrate, or histamine. Other neurotransmitter antagonists like alpha- and beta-adrenoceptor-blocking agents have limited impact on opiate-induced responses. DA antagonists have been found to markedly potentiate many of the central effects of morphine and other opioids, in contrast, direct and indirect DA agonists have reversed the central effects of opioids. Even more importantly in low to moderate doses, DA antagonists that selectively and appreciably inhibit DA release can also mimic many of these effects [30]. In fact, in the neostriatum the biosynthesis of opioid peptides is most dependent on the dopaminergic transmission while, variations in the amplitude of the of mu opioid receptor density and preprodynorphin and preproenkephalin messenger RNA levels, indicate that the regulation, of pallidal and neostriatal mu opioid receptors is more susceptible to a direct opioid antagonism. Haloperidol was found to cause down-regulation of mu opioid receptors that resulted in adaptive changes that increase enkephalin release and biosynthesis [31].
In order to understand the role of opioidergic mechanisms on reward activity we hereby evoke the following information involving the interaction of mu opiate receptor and GABA and most importantly dopaminergic function. In essence then, increased enkephalin with an accompanied reduction of availability of mu-receptors prevents enkephalin attenuation of the GABA-mediated effects on pallidal neuronal activity of dopaminergic denervation and leads to a hypodopaminergic function in the long-term [32]. Simply, reduced mu receptors and as such a reduced enkephalin stimulation of mu receptor inhibition onto GABA receptors at the substania nigra, will lead to a reduced neuronal release of dopamine at the nucleus accumbens. It seems parsimonious then, to consider the potential of combining buprenorphine with a natural D2 agonist which bypasses the GABA inhibition step. A recent study has eloquently shown that if the gene that codes for the β3 subunit of the GABA(A) receptor is removed, DA release, enhanced reward learning and even decision –making, is increased, in electrically stimulated neurons, in the nucleus accumbens of β3-knock out (KO) mice. Specifically DA neurons in midbrain slices from β3-KO mice exhibited attenuated GABA-evoked inhibitory postsynaptic currents (IPSCs). As measured by fast-scan cyclic voltammetry, more DA release into the nucleus accumbens of β3-KO mice was elicited by electrical stimulation of the excitatory afferents to DA neurons. When β3-KO mice were given morphine, most likely resulting in a compensatory upregulation of GABAergic tone onto DA neurons β3-KO mice were more active than controls, in two food-reinforced learning paradigms, they learned faster, and their learned behavior was extinguished normally. Additionally, aversive learning was unchanged in β3-KO while, enhanced learning was specific for appetitive tasks. Finally, Parker et al. [42] found in performance of a probabilistic selection task that required mice to choose between and a large uncertain reward and a small certain reward, β3-KO mice had an enhanced risk preference. These findings collectively identify a selective role in appetitive learning and decision-making for GABA (A) signaling in DA neurons. The clinical relevance, in terms of long term therapy with buprenorphine/naloxone combination, involves the development of a hypodopaminergic state even independent of genetic predisposition, like carrying DRD2 A1 allele with resultant 30-40% reduction in D2 receptor density. This leads to unwanted mood swings and enhanced drug craving and seeking. Moreover, based on an analysis that covered the whole gene locus from the DRD2 promoter to the ANKK1 rs1800497C>T polymorphism DRD2 genetic polymorphisms help to identify those at risk for opiate addiction and to modulate the dosage requirements necessary for methadone or buprenorphine substitution therapy [43].
Brief Description of KB220Z
KB220Z is a formulation that consists of enkephalinase-catecholamine–methyl-transferase (COMT)/monoamine –oxidase (MOA) inhibition therapy and amino-acid neurotransmitter precursors that work, in synchrony, to support brain reward function (Table 1). This is the first neuroadaptive formulation known to activate the brain reward circuitry and is called Neuroadaptagen Amino Acid Therapy™ (NAAT) [44]. Numerous clinical effects that benefit victims of “Reward Deficiency Syndrome” (RDS) are repeatedly confirmed in ongoing research. RDS is a term that describes hypodopaminergic function in the mesolimbic system of the brain. Hypodopaminergic function, can be the trigger for compensatory addictive, compulsive and impulsive behaviors that can elicit extreme DA release that in turn results in DRD2 down -regulation [45]. Gentle activation of brain reward circuitry using NAAT (KB200Z) that has the potential to up –regulate DAD2 receptor density has been demonstrated using qEGG in the United States [46] and in early, unpublished findings in China using fMRI. Victims of Substance Use Disorder (SUD) using a resting state fMRI 2X2 placebo design [44] KB220Z demonstrated both regulation of heroin-induced abnormal connectivity in the putamen, (a site for emotionality) and caudate DA activation. Based on these and 25 earlier clinical trials [44] we decided to evaluate the potential of using KB220Z as a safe natural modality to facilitate recovery from long –term Buprenorphine and Naloxone combination (Table 1).
Withdrawal Related to Sublingual Administration of Suboxone®
It is well-known that withdrawal from intravenous use of buprenorphine/naloxone combination results in severe and difficult withdrawal symptoms [47]. However, less is known about withdrawal related to sublingual administration of Suboxone®. In fact, patients on a dosage of 1-2 mg of buprenorphine experience great difficulty in weaning off of the drug (personal communication by the present authors JF, JB, LB, JG, JAB and CR). Despite clinical evidence from national American Society of Addiction Medicine (ASAM) meetings and other continuing medical education (CME) events, a recent PUBMED search did not reveal any studies describing withdrawal from either long or short term Suboxone®.
Method
This paper presents a description of withdrawal symptoms occurring in a highly addicted patient weaned off long-term buprenorphine/naloxone combination therapy (Suboxone®) and provides clinical support for the use of a natural dopaminergic agonist KB220Z as a putative maintenance adjunct. The genotyping was performed at the Institute of Behavioral Genetics, Colorado University, Boulder, Colorado under the supervision of Dr. Andrew Smolen, using well-known standard genotyping techniques [46]. We genotyped the patient using a 9 reward gene polymorphic (18 allele) panel including: DRD 2,3,4; MOA-A; COMT; DAT1; 5HTTLLR; OPRM1;and GABRA3.
Case Description
Krissie is a 35 year old adopted female residing in California and a former Legal Librarian has signed a consent form allowing her records to be reviewed for this case presentation. In 2002 she began to take prescription opioids for Carpel Tunnel Syndrome (CTS). CTS is a median entrapment neuropathy, that causes paresthesia, pain, numbness, and other symptoms in the distribution of the median nerve due to its compression at the wrist in the carpal tunnel. It is thought that compression of the median nerve traveling through the carpal tunnel causes the pathology. It is caused by a combination of genetic and environmental factors. Some of the known predisposing factors include: diabetes, obesity, pregnancy, hypothyroidism, and heavy manual work or work with vibrating tools but not lighter work even if repetitive.
| GRAS Nutrient Pathway | |
|---|---|
| D-Phenylalanine | Opioid Peptides |
| L-Phenylalanine | Dopamine |
| L-Tryptophane | Serotonin |
| L-Tyrosine | Dopamine |
| L-Glutamine | GABA |
| Chromium | Serotonin |
| Rhodiola Rosea | COMT/MOA |
| Pyridoxine | Enzyme catalyst |
Table 1: Neuroadaptagen Amino-Acid Therapy (NAAT) KB220Z.
In 2003 Krissie underwent Carpel Tunnel surgery. Then in 2005 she was diagnosed with having both severe Fibromyalgia and Reflex Sympathetic Dystrophy. Through Workers Compensation Insurance she was placed under a physician’s care. During the period between 2006 and 2008 the patient was legally prescribed a number of pharmaceuticals (Table 2). It is noteworthy that the monthly workers compensation payout was up to $53,875.63 per month in September 2008.
In 2008 Krissie was treated by a certified Addiction Medicine Physician (ASAM). At this time she was placed on Suboxone® (36 mg) (Table 3).
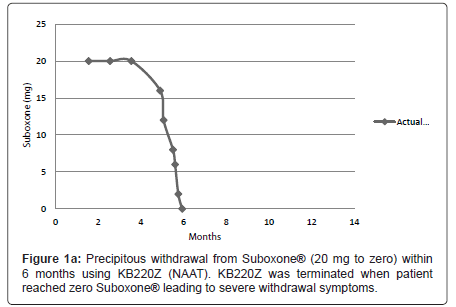
On September 29th 2008 the patient entered the Malibu Recovery Center, Malibu Beach, California. After 73 days she entered the Oceanside Sober Living Center, Oceanside, California for a six (6) week stay then was discharged home. The patient attended both Alcoholics Anonymous and Narcotic Anonymous programs. Along with Suboxone® (32 mg) the patient was still taking anti-migraine medication, Lyrica® and Trazadone® for sleep. Between 2008 and December 17th 2010 dosage was reduced gradually to 20 mg of Suboxone ®. She then entered the out -patient program of Malibu Recovery Center where she received SynaptaGenX™ NAAT (KB220Z) [distributed by Nupathways, Indianapolis, Indiana] tablets (four per day in divided dosage equivalent to 24 gm per day (Table 1). The patient continued to take NAAT until March 28th 2011 when she re-entered the Malibu Beach Recovery Center’s in-patient program. At that point the patient reduced the Suboxone® to 16mg per day. On April 5th Suboxone® was reduced to 12 mg per day. Suboxone® was further reduced on: April 15th to 8mg per day; April 18th to 6 mg per day; on April 22nd to 2mg per day. Then finally on April 28th Suboxone® was reduced to zero mg per day (Figure 1a), when due to the severity of the withdrawal symptoms the patient decided not to take the NAAT. The severity of withdrawal became clinically relevant when she dropped from 2 mg and below. The withdrawal symptoms continued quite severely for at least one week following complete drug termination.
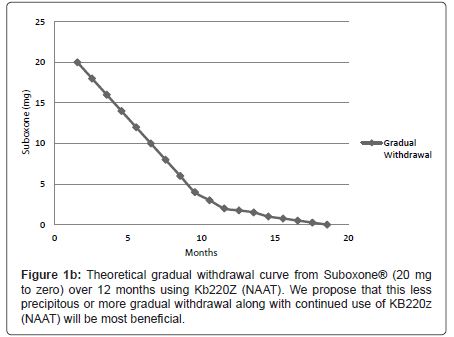
We are proposing that a gradual withdrawal over a longer time frame and continued utilization of KB220Z (NAAT) would potentially facilitate an easier withdrawal from Suboxone® (Figures 1a and 1b). Based on previous clinical experience (JF) no greater than 5-10% reduction every few weeks should result in only minimal withdrawal symptoms. However if withdrawal symptoms do return the dose of Suboxone is held until symptoms resolve and only then does taper resume.
The withdrawal symptoms as self –described by the patient were severe (Table 4). The patient did not take NAAT again until May 6th 2011 after the withdrawal symptoms had subsided. On May 11th 2011 the patient was discharged from the residential treatment center and continued taking NAAT four tablets per day.
| Medication Name | Strength | Directions | Qty | Cost | Notes |
|---|---|---|---|---|---|
| Methadone | 10 mg | 2 tabs every 6 hours | 240 | $56.50 | None |
| Morphine | 100 mg | 1 or 2 tabs every 6h PRN pain | 240 | $228.80 | Assumes SR formulation |
| Oxycodone | 30 mg | 1 or 2 tabs every 4-6h PRN pain | 360 | $48.60 | None |
| Flurazepam | 30 mg | 1cap at bedtime | 30 | $15.00 | None |
| Clonazepam | 2 mg | 1 tab TID PRN anxiety | 90 | $24.95 | None |
| Duragesic (Fentanyl) | 100 mcg | 3 patches at a time, switching one every day | 30 | $2,818.89 | Brand name costs & assumes 100mcg strength |
| Amitiza | 24 mcg | 1 cap BID | 60 | $ 254.00 | None |
| Tizanidine | 4 mg | 1 tab TID, 3 tabs at night | 180 | $ 45.69 | None |
| Trazodone | 100 mg | BID | 60 | $15.00 | None |
| Actiq | 1600 mcg | 12-14/day | 420 | $49,985.80 | Brand name costs |
| Provigil | 200 mg | BID | 60 | $382.40 | None |
| Costs | |||||
| Total Cost per month | $ 53,875.63 |
Table 2: Monthly medications (narcotics and benzodiazepines and workers compensation cost in september 2008.
| Suboxone | 8 mg | Take 4 ½ tablets (36 mg) SL daily | 140 | $ 1,000 | Based on current costs |
Table 3: Monthly medications (Suboxone) and workers compensation cost.
| Case 1. Long-term Suboxone Detoxification Symptoms |
|---|
| •creepy crawlies; •Heebie Jeebies; •leg shaking; •excruciating pain all over the body; •stomach upset; •cramps, •knotted, •nausea, •vomiting; •diarrhea; •headaches; •fever blisters on mouth; •uncomfortable in my own skin; •felt that she was out of mind, •hallucinations (seeing ghosts); •chills, •dizziness. |
Table 4: Withdrawal symptoms from long Suboxone® therapy.
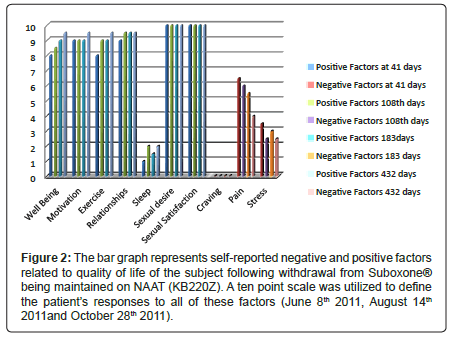
Figure 2: The bar graph represents self-reported negative and positive factors related to quality of life of the subject following withdrawal from Suboxone├?┬« being maintained on NAAT (KB220Z). A ten point scale was utilized to define the patient├ó┬?┬?s responses to all of these factors (June 8th 2011, August 14th 2011and October 28th 2011).
As of June 8th 2011 (41 days since withdrawal on April 28th) in a structured one hour clinical interview the patient reported the following using a ten point scale: overall well being was at a 8 (10 being the highest positive well-being score); motivation for job seeking was at a 10; walking and exercise was at 8; happiness in marriage was at a 9; sexual desire 10; sexual experience 10; poor sleep at around 1; cravings for any psychoactive drug was zero; pain but tolerable at about 6.5 level and stress level was at a 3 level. On August 14th (108 days post dose cessation on April 28th 2011) we followed up with another structured interview with the patient who provided rating using a ten point scale: overall –well being 8.5; Motivation 9; Exercise 9; relationships 9.5; sleep 2; sexual desire 10; sexual satisfaction 10; craving 0; pain 6 and stress 2.5.
On October 28th 2011 (after 183 days) we followed up with another structured interview with the patient who provided rating using a ten –point scale: Overall-well-being 9; Motivation 9; Exercise 9; relationships 9.5; sleep 1.5; sexual desire 10; sexual satisfaction 10; craving 0; pain 5.5; stress 3.0 (Figure 2). The same results were obtained on 12/13/11 (after 228 days) and as such were not included in Figure 2. Krissie has also reported a remarkable reduction in glucose cravings with an associated loss of 35 pounds, achieving her healthy weight reduction goal, following withdrawal from Suboxone® with KB200Z™ maintainence.
Currently we are addressing the sleep issues and the pain tolerance condition and the patient will continue to be monitored. The patient was admitted to a sleep clinic in Los Angeles on 10/20/11 where they discovered abnormal EEG sleep patterns. Krissie reached stage 2 REM sleep after a 3 hour period and did not reach delta sleep in the test period from 8:00 pm to 6:00 am.
On July 14th 2011 the patients was urine tested negative for all opioids, psychostimulants, depressants, marijuana and alcohol. This was confirmed by Redwood Toxicology Laboratory (RTL), Santa Rosa, California. According to Mark J. Demeo, MD in accordance with all RTL standard operating procedures, opiates were not detected in the specimen. On December 13th a urine test was performed and reported on December 21st 2011 by Wayne Ross. The patient was negative for the following: alcohol, barbiturates, oxycodone/noroxcodone, phencyclidine (PCP), buprenorphine, cocaine (benzoylecgonline), THC and amphetamines. However the patient was positive for prescribed cough medication. It is our clinical opinion that since the cough medicine was prescribed by her physician without realizing her past history of prescription opioid addiction and she admitted it to us prior to testing we do not believe that this constitutes relapse. As of February 25th 2012 the patient no longer uses the cough medicine and is completely off benzodiazepines. On June 15th the patient was once again urine tested and was opiate free. One arguable theoretical retort is that since fentanyl was not specifically urine tested the patient could be continuing her opioid utilization by covertly obtaining fentanyl through street diversion. However, the patient absolutely reports being completely drug free including fentanyl.
Genotyping Results Guide Treatment
Our subject was genotyped for nine well characterized reward candidate genes (Table 5) and for females 18 alleles. The results revealed a polymorphism 4 R/4R (homozygous) which relates to a high MOA-A activity which could lead to a more rapid degradation of Dopamine (DA) in the mitochondria once the released DA is sequestered back into the pre-synaptic neuron. Genotyping also revealed polymorphisms in the dopamine transporter gene (DAT1) whereby she carried homozygote 9R/ 9R which suggests that her transporter system is highly active and as such sequesters higher amounts of DA ( higher uptake) to re-enter the pre-synaptic neuron as well. Consistent with these findings the subject also carries the heterozygous A/G (Val) polymorphism which will result in a higher activity of COMT and as such the DA in the synapse will be broken down at a faster rate. Taken together these results strongly suggest a “hypodopaminergic state”. Along with this proposed “hypodopaminergic state” (or genetic trait) the subject also is heterogeneous for polymorphism in the GABA receptor beta subunit (183) which could result in reduced GABA sensitivity leading to anxiousness or stress. Genotyping of the DA receptors (D2,3,4) were found to be normal in terms of receptor densities as well as the Mu opiate receptor. In addition, she possesses the homozygote serotonin transporter L/L maybe protective against alcohol seeking behavior.
| GENE/ALLELE | RESULT |
|---|---|
| Caspi MAOA uVNTR | 4R |
| Caspi MAOA uVNTR | 4R |
| DRD4 | 4R |
| DRD4 | 4R |
| DAT | 9R |
| DAT | 9R |
| 5HTTLLR dialletic | L/L |
| COMT | A/G |
| DRD2 Taq1 | A2/A2 |
| DRD3 C=Gly T=Ser | T/T |
| OPRM1 A=Asn G=Asp | A/A |
Table 5: GARS result panel.
Understanding this important genotyping information provides impetus to development of a protocol that converts her low dopaminergic state to a higher dopaminergic state. An increase in synaptic DA can be facilitated by the ingredients in KB220Z. Rhodiola rosea is known to block MOA and COMT activity and both phenylalanine and tyrosine impede the high activity of the molecules involved in DAT gate (leading a reduced DAT activity). Further it is conjectured that enhanced quanta of DA could offset the anxiety due to GABA polymorphisms that result in reduced DA and as such increased norepinephrine activity (stress molecule). This mechanism may help us explain the 432 opioid free days which maybe attributable to the neurochemical effects of KB220Z.
In terms of presentation Krissie (patient) has provided clearance and consent to show her likeness pre and post treatment and precipitous withdrawal from Suboxone. The pre –likeness is upon first entry to Malibu Recovery Treatment Center whereas the post –likeness is the most recent image taken 432 days on NAAT [KB220Z] (Figure 3).
Discussion
These preliminary findings provide the first ever evidence for the clinical utilization of a natural dopaminergic activator to wean patients from long-term buprenorphine/naloxone combination therapy in chemical dependency treatment facilities and programs. It is quite possible that after a severe withdrawal reaction due to such a rapid tapering the short term resolve of these symptoms had nothing to do with KB220Z. However, the long –term benefits reported herein may be due the known activation of dopaminergic pathways by KB220Z [48]. It is suggested that the overall successful symptom resolution may be due to strengthening of dopaminergic activity augmenting relapse prevention.
In this case, Krissie suffered severe chronic pain due to both severe Fibromyalgia and Reflex Sympathetic Dystrophy following Carpel Tunnel surgery. The patient had been on buprenorphine/naloxone for over two and one-half-years. At the time of admission to the Malibu Recovery Center, the patient was on 20 mg of the combination therapy. Prior to detoxification (weaning) the patient took KB220Z for a three month period. Rapid reduction of the buprenorphine/naloxone combination began on March 28th 2011 and ended April 28th, 2011. While the withdrawal process was difficult the patient is currently taking only KB220Z and Lyrica. While the pain continues it is bearable with KB220Z due to its ability to activate dopaminergic pathways. Dopamine tone is involved in the inhibition of inflammation and chronic pain [49]. Tonic pain inhibition is regulated by activated mesolimbic dopamine neurons that project into the nucleus accumbens from the cell bodies of the ventral tegmental area [50]. When this pain-suppression system is activated by acute stress endogenous opioids and substance P are released. However, a reduction of nucleus accumbens dopamine output and development of persistent hyperalgesia are produces by prolonged exposure to unavoidable stress. Krissie self-reported that she experienced chronic long-term stress which certainly affected her overall pain [51]. It is noteworthy that KB220Z variant, in double-blind placebo controlled studies using skin conductance to objectively measure stress, significantly reduced the stress response in poly-drug abusers [52]. It must be stated that of cause the reduction of suffering from pain may –in-part be also due to the removal of hyper-analgesia due to withdrawal from opioids. Clinicians have observed that patients overall degree of pain stay in the 4-6 range, but the pain is more tolerable, and the patient is more functional.
D-phenylalanine (D- Pha) a component of KB200Z acts as an analgesic due to its known enkephalinase inhibition properties [53]. Moreover, Litvinova et al. [54] found in morphine-sensitive (s.c. 1.5 mg/kg) Wistar rats (60%) I. P. inoculation of 300-600 mg/kg D-Pha did not change the tail-flick test nociception, but did evoke a dose-dependent analgesic effect in morphine-resistant rats (40%). In morphine-sensitive rats (40%) chronic morphine administration induced the tolerance and D-Pha injection evoked an analgesic effect. Morphine injection just after D-Pha over-evoked an analgesic effect in morphine-resistant and morphine-tolerant rats. It is suggested that morphine tolerant rats have acquired and morphine-resistant rats have a congenital high level of enkephalinase activity which blocked the morphine analgesic action.
In addition there is clear evidence that KB220Z significantly influences drug-induced withdrawal symptoms [55], building up to relapse [56], leaving against medical advice (AMA rates) [57] drug hunger [58], regulation of dysregulated widespread delta waves in psychostimulant abusers [46] as well as alcohol and opiate addicts [59] and an enhanced happiness [60]. The patient utilized KB220Z for approximately six weeks prior to withdrawal and maintained its use up to April 28th when the patient experienced severe withdrawal from coming off of the 2 mg dose of Suboxone®. It is reasonable to consider that since the patient did not experience significant mini withdrawal symptoms from the rapid tapering to the 2 mg dose, normally observed clinically in most patients (i.e. mood swings, low energy, poor sleep, GI upset etc) the utilization of KB220Z could have attenuated the mini withdrawal symptoms. This observation is the subject of a larger proposed study. Unfortunately due to the terrible gastrointestinal upset the patient could not tolerate KB220Z. This resulted in a very unpleasant detoxification which lasted for at least 6 days post Suboxone.® In retrospect we are proposing a smoother detoxification that should include a much longer period for titration of the Suboxone® Moreover in a needed larger study we will evaluate the use of KB220IV during the actual withdrawal period. Additionally we conjecture that oral KB220Z [61] in combination with acupuncture [62] should significantly reduce the withdrawal severity (Figure 2). While numerous studies support the efficacy of opiate agonist treatment with either methadone or buprenorphine the question of severe withdrawal from these opiate agonistic drugs cannot be ignored in clinical practice [63-68]. Moreover, neuroimaging studies of buprenorphine/naloxone combinations do not support an effect on cingulate-gyrus activation. The cingulate-gyrus is thought to be the loci of relapse prevention [69].
It is noteworthy that Krissie continues to successfully attain well-being and enhanced quality of life utilizing oral KB220Z (432 days after detoxification from Suboxone®). However, the cause of the prolonged sleep disturbance remains a mystery and is an impediment to complete recovery.
Based on this case study we cautiously suggest that our laboratory may have discovered a new side effect free and cost effective (> $100 per month) modality to assist in the attenuation of patients from the buprenorphine/naloxone combination therapy following detoxification and drug use cessation programs.
Patients with SUD and those like Krissie who have become addicted to legal prescription anti-pain medication [70] are increasingly placed on the buprenorphine/naloxone combination. As of 2008 the total patient population using buprenorphine was 649,550 based on the federal Center for Substance Abuse Treatment [CSAT] [10]. If an intervention that consisted of neuro-therapy to reduce pain and the use of neuroadpatgen-amino-acid –therapy (NAAT), to enhance “dopamine sensitivity”, could attenuate pain at (10-60%) the savings may be counted in billions. Such a program could consist, for example, of KB220Z; cranial stimulation, electrotherapy for pain control and biofeedback.
Certainly, clinicians are beginning to understand long term effects of Suboxone and potential induction of a flat affect [71] and as such are reducing the amount of Suboxone they prescribe even in chronic pain clinics (personal communication Gary Reisfield). We are cognizant that many large required clinical studies are mandatory before the above scenario becomes evident but it is at least a plausible outcome for the future.
It is noteworthy that one contributor to buprenorphine withdrawal syndrome are the high dosages used for Suboxone/Subutex ORT. Given that buprenorphine is about 40 times more potent than morphine, each 2 mg capsule or film is the equivalent of 80 mg Morphine sulfate. In fact sublingual (SL) buprenorphine is often referred to as “high dose buprenorphine”. So even if you stop buprenorphine at 1/2 the smallest dosage form, that is still like stopping Kadian (oral morphine) 20 mg two-times a day (BID) abruptly without a wean, though with buprenorphine’ long half- life it may be less intense but last longer similar to methadone withdrawal. We must underscore the clinical fact that even patients coming off of ¼ mg of Suboxone ® have severe withdrawal. One of us (JF) believes that is not the rate of taper but the rate of reactivation of reward deficit.
Recently, the 7 day Butrans® patch became available, and is FDA approved for pain. The highest dosage it comes in is 20 mcg/hour; this is less than 1/2 mg per day. One strategy that could be used is to wean SL buprenorphine to 1 mg/day, then change over to the Butrans patch weaning from 20 to 15 to 10 mcg/h, though it would take some time (Figure 1b) and insurance does not typically cover it. So there may not be anything special about Buprenorphine withdrawal as compared to other opioids other than the incredibly high dosages of Suboxone. The smallest pill/film available contains 2 mg SL buprenorphine, although it is the smallest dosage, it is actually a very high opioid dosage.
Krissie was actually detoxifying from a high dose of 20 mg of Suboxone that she was taking for many months prior to her rapid tapering. It has been clinically documented that patients undergoing rapid tapering morphs the chance of neuroadaptations and reactivation of the reward process. This leads to persistence in sleep disturbance, low energy and altered mood. While these symptoms could be relieved by opioid replacement we are interested in other alternative therapies, especially those that could naturally activate caudate-accumbens dopaminergic pathways.
Looking to the future of pain regulation it may be parsimonious to include genetic testing prior to prescribing powerful analgesic opioid pharmaceuticals. That the principal ascending pathways for pain originate in the spinal cord at the dorsal horn and in the medulla is well known, however, the neurological control and sensitivity to pain may reside in the reward center of the brain, the mesolimbic system. There a number of genes and their polymorphisms associate with a predisposition to tolerance or intolerance to pain. Unique therapeutic target to assist in the treatment of pain may result from the identification of certain gene polymorphisms. It is hereby further proposed [50] that pharmacogenetic testing of certain candidate genes (i.e., cytochrome P450 (CYP) family, mu receptors, PENK etc.) will result in pharmacogenomic solutions personalized to the individual patient, with potential for improvement in clinical outcomes and reduction of iatrogenic induced drug addiction.
Relapse prevention is supported by KB200Z
Unlike buprenorphine/naloxone combination [69] which does not activate the cingulate gyrus (a site for drugs abuse relapse) KB220Z activates the cingulate gyrus and induces regulation of aberrant electrical dysregulation (widespread theta) in the cingulated gyrus as measured by qEEG analysis [48,59]. These results support our earlier reports [44], that KB220Z/KK220IV are natural dopaminergic agonists and have potential as therapeutic agents for weaning, relapse prevention [56], and to provide benefit in opioid dependence –Suboxone® treatment programs as an adjunct to therapy in the short term [72] and merit further intensive investigation.
It is noteworthy, that most patients don’t relapse after a few years because of cue reactivity originating in PFC-cingulate gyrus and possibly disruption of executive functioning [73] instead it is usually due to stress, re-exposure to narcotics, lowering of the dose of non-drug recovery equivalents and complacency.
Recently, Diana [74] pointed out that imaging studies visualized reduced dopamine receptor density accompanied by decreased release of endogenous dopamine in the ventral striatum of alcohol, cocaine and heroin dependent subjects, proof of dopamine deficiency in drug dependant subjects. This new objective study supports the concept of reward deficiency syndrome [45] and its treatment with dopamine agonist therapy to reduce cravings and prevent relapse and drug seeking behavior. It is noteworthy, that when Doehring et al. [43] analyzed the whole DRD2 gene locus they found that DRD2 genetic polymorphisms regulate both opiate addiction risk and ORT dosage requirements. Relevant to this article Seifert et al. [27] found that compared to carbamazepine and methadone, patients on the buprenorphine and carbamazepine combination demonstrated significantly better psychological state with reduced, sensitiveness, tiredness and depression by week two of detoxification. Once again this work adequately supports the short term use of buprenorphine.
This case study requires follow-up with a much larger population using controlled blinded conditions in order to make any definitive statements. Our laboratory is actively pursuing a much larger sample size and in fact unpublished we are finding similar benefits when KB220Z is combined with Suboxone® in outpatient heroin addicts. However important information has been gleaned from this pilot.
Limitations of the Study
Since this study involves only one patient we cannot provide any definitive conclusions at this juncture. We are encouraged that KB220Z positively enhances the quality of life factors while reducing negative factors such as cravings and stress following 432 days post Suboxone® withdrawal. According to the patient despite the problem with sleep, KB220Z- “saved her life”.
Obviously the main limitation of the results herein is that it is a single case study. Certainly, in spite of these interesting documented findings, we are cognizant that potentially without KB220Z some if not all of these benefits may have occurred through natural recovery process and larger controlled studies are required. Additionally one could easily argue that without intensive and repeated urine testing the patient could be utilizing opioids through diversionary tactics.
Conclusion
To our knowledge this is the first peer reviewed report describing severe withdrawal reactions (Table 4) following long –term (2.5 yrs) use on Suboxone® therapy. Secondly, it is the first report providing some evidence for the use of KB220Z as an appropriate non-addicting substitution therapy for opioid dependence. The daily use of KB220Z, a natural dopamine agonist neuroadaptagen showing dopaminergic activation properties as measured by qEEG and fMRI, provides an alternative substitution adjunctive modality influencing brain reward circuitry [44,46,59,75-83]. We encourage additional double-blind randomized –placebo controlled studies to test the proposition that KB220Z may act as a putative natural opioid substitution maintenance adjunct.
Consent
Written informed consent was obtained from the patient for the publication of this case study report and the accompanying data. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
SynaptaGenX™ the liposomal form was supplied by Nupathways Inc. Indianapolis Indiana. Controlled by U.S. patent # 6,132,724 licensed to LifeGen, Inc. Support to MOB comes from NIAAA grants ROI –AAO7112and K05-AA00219, and the Medical Research Services of the VA.
Conflict of Interest
Kenneth Blum, B. William Downs, Roger L. Waite and Margaret Madigan own stock in LifeGen, Inc. the worldwide exclusive licensee of US and foreign patents issued and pending related to KB220 variants marketed under the commercial name of SynaptaGenX™. John Giordano is a partner with LifeGen, Inc. Mary Hauser is an officer of Dominion Diagnostics a company that is involved with the commercialization along with LifeGen, Inc. of the Genetic Addiction Risk Score (GARS) test. Kenneth Blum is a paid consultant on the Scientific Advisory Board of the Dominion Diagnostic. Joan Borsten, Kenneth Blum, B.W. Downs, Roger L. Waite and Margaret A. Madigan have interest in RXPAIN™. There are no other conflicts of interest.
References
- Wood E, Li K, Palepu A, Marsh DC, Schechter MT, et al. (2005) Sociodemographic disparities in access to addiction treatment among a cohort of Vancouver injection drug users. Subst Use Misuse 40: 1153-1167.
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, et al. (2005) Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry 162: 340-349.
- Dole VP, Nyswander ME (1966) Rehabilitation of heroin addicts after blockade with methadone. N Y State J Med 66: 2011-2017.
- Goldstein A (1991) Heroin addiction: neurobiology, pharmacology, and policy. J Psychoactive Drugs 23: 123-133.
- Butler M, RL Kane, D McAlpine, RG Kathol, SS Fu, et al. (2008) Integration of Mental Health/ Substance Abuse and Primary Care No. 173 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009.) AHRQ Publication No. 09-E003. Rockville, MD. Agency for Healthcare Research and Quality.
- US Department of Health and Human Services (2003). Substance Abuse and Mental Health Services Administration (SAMHSA): Opioid agonist therapy (OAT) with methadone or LAAM.
- Ling W (2009) Buprenorphine for opioid dependence. Expert Rev Neurother 9: 609-616.
- Judson BA, Goldstein A (1984) Naltrexone treatment of heroin addiction: one-year follow-up. Drug Alcohol Depend 13: 357-365.
- Blum K, Futterman S, Wallace JE, Schwertner HA (1977) Naloxone-induced inhibition of ethanol dependence in mice. Nature 265: 49-51.
- Arfken CL, Johanson CE, di Menza S, Schuster CR (2010) Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat 39: 96-104.
- Wesson DR, Smith DE (2010) Buprenorphine in the treatment of opiate dependence. J Psychoactive Drugs 42: 161-175.
- McNicholas L (2004) SAMHSA clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol, 40. U.S. Department of Health and Human Services, Rockville, MD.
- Blum K, Chen TJ, Bailey J, Bowirrat A, Femino J, et al (2011) Can the Chronic Administration of the Combination of Buprenorphine and Naloxone Block Dopaminergic Activity Causing Anti-reward and Relapse Potential? Mol Neurobiol 44: 250-268.
- Farrell M, Wodak A, Gowing L (2012) Maintenance drugs to treat opioid dependence. BMJ 344: e2823.
- Jasinski DR, Pevnick JS, Griffith JD (1978) Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 35: 501-516.
- Umbricht A, Montoya ID, Hoover DR, Demuth KL, Chiang CT, et al. (1999) Naltrexone shortened opioid detoxification with buprenorphine. Drug Alcohol Depend 56: 181-190.
- Elkader A, Sproule B (2005) Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet 44: 661-680.
- Elkader A, Sproule B (2005) Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet 44: 661-680.
- Fang WB, Chang Y, McCance-Katz EF, Moody DE (2009) Determination of naloxone and nornaloxone (noroxymorphone) by high-performance liquid chromatography-electrospray ionization- tandem mass spectrometry. J Anal Toxicol 33: 409-417.
- Bruce RD, Altice FL, Moody DE, Morse GD, Andrews L, et al. (2010) Pharmacokinetic interactions between buprenorphine/naloxone and once-daily lopinavir/ritonavir. J Acquir Immune Defic Syndr 54: 511-514.
- Orman JS, Keating GM (2009) Spotlight on buprenorphine/naloxone in the treatment of opioid dependence. CNS Drugs 23: 899-902.
- Harris DS, Jones RT, Welm S, Upton RA, Lin E, et al. (2000) Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend 61: 85-94.
- Ciraulo DA, Hitzemann RJ, Somoza E, Knapp CM, Rotrosen J, et al. (2006) Pharmacokinetics and pharmacodynamics of multiple sublingual buprenorphine tablets in dose-escalation trials. J Clin Pharmacol 46: 179-192.
- Kosarac B, Fox AA, Collard CD (2009) Effect of genetic factors on opioid action. Curr Opin Anaesthesiol 22: 476-482.
- Zhou SF, Liu JP, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41: 89-295.
- Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN (1998) Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend 50: 1-8.
- Seifert J, Metzner C, Paetzold W, Borsutzky M, Ohlmeier M, et al. (2005) Mood and affect during detoxification of opiate addicts: a comparison of buprenorphine versus methadone. Addict Biol 10: 157-164.
- Winslow JT, Miczek KA (1988) Naltrexone blocks amphetamine-induced hyperactivity, but not disruption of social and agonistic behavior in mice and squirrel monkeys. Psychopharmacology (Berl) 96: 493-499.
- Gold MS (1993) Opiate addiction and the locus coeruleus. The clinical utility of clonidine, naltrexone, methadone, and buprenorphine. Psychiatr Clin North Am 16: 61-73.
- Hume SP, Lingford-Hughes AR, Nataf V, Hirani E, Ahmad R, et al. (2007) Low sensitivity of the positron emission tomography ligand [11C]diprenorphine to agonist opiates. J Pharmacol Exp Ther 322: 661-667.
- Feigenbaum J, Yanai J (1984) The role of dopaminergic mechanisms in mediating the central behavioral effects of morphine in rodents. Neuropsychobiology 11: 98-105.
- Mavridis M, Besson MJ (1999) Dopamine-opiate interaction in the regulation of neostriatal and pallidal neuronal activity as assessed by opioid precursor peptides and glutamate decarboxylase messenger RNA expression. Neuroscience 92: 945-966.
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA (2011) GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience 172: 94-103.
- Galynker I, Schlyer DJ, Dewey SL, Fowler JS, Logan J, et al. (1996) Opioid receptor imaging and displacement studies with [6-O-[11C] methyl]buprenorphine in baboon brain. Nucl Med Biol 23: 325-331.
- Darke S, Ross J, Lynskey M, Teesson M (2004) Attempted suicide among entrants to three treatment modalities for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence and risk factors. Drug Alcohol Depend 73: 1-10.
- Hervé S, Riachi G, Noblet C, Guillement N, Tanasescu S, et al. (2004) Acute hepatitis due to buprenorphine administration. Eur J Gastroenterol Hepatol 16: 1033-1037.
- Peyrière H, Tatem L, Bories C, Pageaux GP, Blayac JP, et al. (2009) Hepatitis after intravenous injection of sublingual buprenorphine in acute hepatitis C carriers: report of two cases of disappearance of viral replication after acute hepatitis. Ann Pharmacother 43: 973-977.
- Taylor LE, Maynard MA, Friedmann PD, Macleod CJ, Rich JD, et al. (2012) Buprenorphine for human immunodeficiency virus/hepatitis C virus-coinfected patients: does it serve as a bridge to hepatitis C virus therapy? J Addict Med 6: 179-185.
- Gaulier JM, Marquet P, Lacassie E, Dupuy JL, Lachatre G (2000) Fatal intoxication following self-administration of a massive dose of buprenorphine. J Forensic Sci 45: 226-228.
- Kintz P (2001) Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int 121: 65-69.
- Tracqui A, Tournoud C, Flesch F, Kopferschmitt J, Kintz P, et al. (1998) [Acute poisoning during substitution therapy based on high-dosage buprenorphine. 29 clinical cases--20 fatal cases]. Presse Med 27: 557-561.
- Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, et al. (2011) Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci 31: 17103-17112.
- Doehring A, Hentig Nv, Graff J, Salamat S, Schmidt M, et al. (2009) Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics 19: 407-414.
- Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, et al. (2011) Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by amino-acid precursor-catabolic enzyme inhibition therapeutic agent (a Neuroadaptagen): Proposing an addiction candidate gene panel map. J Psychoactive Drugs 43: 108-127.
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, et al. (2000) Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 32 Suppl: i-iv, 1-112.
- Blum K, Chen TJ, Morse S, Giordano J, Chen AL (2010) Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: part 2. Postgrad Med 122: 214-226.
- Gourarier L, Lowenstein W, Gisselbrecht M, Chauveau JM, Haas C, et al. (1996) [Withdrawal syndrome in 2 drug addicts after intravenous injection of buprenorphine?]. Presse Med 25: 1239-1240.
- Blum K, Stice E, Liu Y, Giordano J, Morse S, et al. (2011) “Dopamine Resistance” in brain reward circuitry as a function of DRD2 gene receptor polymorphisms in RDS: Synaptamine complex variant (KB220) induced “Dopamine Sensitivity” and enhancement of happiness. XIX World Congress of Psychiatric Genetics, Washington DC.
- Taylor BK, Joshi C, Uppal H (2003) Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res 987: 135-143.
- Magnusson JE, Fisher K (2000) The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res 855: 260-266.
- Wood PB (2004) Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses 62: 420-424.
- Blum, K, Chen ALC, Chen TJH (2009) Putative targeting of dopamine D2 receptor function in reward deficiency syndrome (RDS) by Synaptamine Complex Variant (KB220): Clinical trial showing anti-anxiety effects. Gene Therapy & Molecular Biology 13: 214-230.
- Chen AL, Chen TJ, Waite RL, Reinking J, Tung HL, et al. (2009) Hypothesizing that brain reward circuitry genes are genetic antecedents of pain sensitivity and critical diagnostic and pharmacogenomic treatment targets for chronic pain conditions. Med Hypotheses 72: 14-22.
- Litvinova SV, Kozlov AIu, Kaliuzhnyi LV (1993) The enkephalinase mechanisms of the resistance and tolerance to the analgesic effect of morphine in rats. Differences in the effects of the action of D-phenylalanine in morphine-sensitive, morphine-tolerant and morphine-resistant rats. Biull Eksp Biol Med 116: 54-56.
- Blum K, Trachtenberg MC, Ramsay JC (1988) Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: a pilot study. Int J Addict 23: 991-998.
- Brown RJ, Blum K, Trachtenberg MC (1990) Neurodynamics of relapse prevention: a neuronutrient approach to outpatient DUI offenders. J Psychoactive Drugs 22: 173-187.
- Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, et al. (1988) Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: Double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol 5: 481-93.
- Blum K, Allison D, Trachtenberg MC, Williams RW, Loeblich LA (1988) Reduction of both drug hunger and withdrawal against advice rate of cocaine abusers in a 30 day inpatient treatment program by the neuronutrient Tropamine. Current Therapeutic Research 43: 1204-1214.
- Miller DK, Bowirrat A, Manka M, Miller M, Stokes S (2010) Acute intravenous synaptamine complex variant KB220™ "normalizes" neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports. Postgrad Med 122: 88-213.
- Blum K, Chen AL, Chen TJ, Rhoades P, Prihoda TJ, et al. (2008) LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv Ther 25: 894-913.
- Blum K, Chen TJH, Downs BW, Meshkin B, Blum SH, et al. (2007) Synaptamine (SG8839) an amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of reward deficiency syndrome (RDS). Trends in Applied Sciences Research 2: 132-138.
- Blum K, Giordano J, Morse S, Anderson A, Carbajal J, et al. (2011) Hypothesizing Synergy between Acupuncture/ Auriculotherapy and Natural Activation of Mesolimbic Dopaminergic Pathways: Putative Natural Treatment Modalities for the Reduction of Drug Hunger and Relapse. IIOAB Letters, North America.
- Quest TL, Merrill JO, Roll J, Saxon AJ, Rosenblatt RA (2012) Buprenorphine therapy for opioid addiction in rural Washington: the experience of the early adopters. J Opioid Manag 8: 29-38.
- Reisfield GM, Sloan PA (2012) Physician identification of opioid diversion: a difficult diagnosis. J Opioid Manag 8: 5-6.
- Balhara YP, Jain R (2012) Urinalysis-based comparative evaluation of pattern of use of dextropropoxyphene and buprenorphine among opioid-dependent subjects. J Opioid Manag 8: 45-49.
- Yokell MA, Zaller ND, Green TC, McKenzie M, Rich JD (2012) Intravenous use of illicit buprenorphine/naloxone to reverse an acute heroin overdose. J Opioid Manag 8: 63-66.
- Rosenblum A, Cruciani RA, Strain EC, Cleland CM, Joseph H, et al. (2012) Sublingual buprenorphine/naloxone for chronic pain in at-risk patients: development and pilot test of a clinical protocol. J Opioid Manag 8: 369-382.
- Weiner M, Sarantopoulos C, Gordon E (2012) Transdermal buprenorphine controls central neuropathic pain. J Opioid Manag 8: 414-415.
- Mei W, Zhang JX, Xiao Z (2010) Acute effects of sublingual buprenorphine on brain responses to heroin-related cues in early-abstinent heroin addicts: an uncontrolled trial. Neuroscience 170: 808-815.
- Stock C, Shum JH (2004) Bupreorphine:a new pharmacotherapy for opioid addictions treatment. J Pain Palliat Care Pharmacother 18: 35-54.
- Hill E, Dumouchel P, Moehs C (2011) "An evidence-based toolset to capture, measure and assess emotional health." In Studies in Health Technology and Informatics, Annual Review of Cybertherapy and Telemedicine 2011 - Advanced Technologies in Behavioral, Social and Neurosciences, p:176-181.
- Arfken CL, Johanson CE, di Menza S, Schuster CR (2010) Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat 39: 96-104.
- Bowirrat A, Chen TJ, Oscar-Berman M, Madigan M, Chen AL, et al. (2012) Neuropsychopharmacology and neurogenetic aspects of executive functioning: should reward gene polymorphisms constitute a diagnostic tool to identify individuals at risk for impaired judgment? Mol Neurobiol 45: 298-313.
- Diana M (2011) The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2: 64.
- Blum K, Wallace JE, Schwerter HA, Eubanks JD (1976) Morphine suppression of ethanol withdrawal in mice. Experientia 32: 79-82.
- Blum K, Briggs AH, DeLallo L (1983) Clonidine enhancement of ethanol withdrawal in mice. Subst Alcohol Actions Misuse 4: 59-63.
- Blum K, Chen TJ, Downs BW, Bowirrat A, Waite RL, et al. (2009) Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing "deprivation-amplification relapse therapy" (DART). Postgrad Med 121: 176-196.
- Blum K, Gold MS (2011) Neuro-chemical activation of brain reward meso-limbic circuitry is associated with relapse prevention and drug hunger: a hypothesis. Med Hypotheses 76: 576-584.
- Blum K, Liu Y, Shriner R, Gold MS (2011) Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des 17: 1158-1167.
- Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment, Division of Pharmacologic Therapies. Diversion and Abuse of Buprenorphine: A Brief Assessment of Emerging Indicators.
- Information on buprenorphine, the DATA 2000 Act, and other aspects of buprenorphine therapy: CSAT Buprenorphine Information Center.
- Monte AA, Mandell T, Wilford BB, Tennyson J, Boyer EW (2009) Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J Addict Dis 28: 226-231.
- JR Havens, M Lofwall, CG Leukefeld Individual and network determinants of buprenorphine misuse among rural prescription opioid users. CPDD 73rd Annual Meeting, Hollywood, Florida.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 21942
- [From(publication date):
June-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 17259
- PDF downloads : 4683