Letter to Editor Open Access
What can be done to Improve Uptake of Genetic Testing for Inherited Colorectal Cancer Susceptibility?
Henry T Lynch MD1*, Carrie Snyder MSN1, Trudy Shaw MA1 and Patrick Lynch JD2
1Department of Preventive Medicine and Public Health, Creighton University, Omaha, NE, USA
2Department of Gastroenterology, Hepatology and Nutrition, University of Texas MD Anderson Cancer Center, Houston, TX, USA
- Corresponding Author:
- Henry T Lynch
Department of Preventive Medicine and
Public Health Creighton University
2500 California Plaza, Omaha, NE 68178, USA
Tel: +1-402-280-2942
Fax: +1-402-280-1734
E-mail: htlynch@creighton.edu
Received Date: May 21, 2013; Accepted Date: November 28, 2013; Published Date: December 05, 2013
Citation: Henry T Lynch MD, Carrie Snyder MSN, Trudy Shaw MA, Patrick Lynch JD (2013) What can be done to Improve Uptake of Genetic Testing for Inherited Colorectal Cancer Susceptibility? J Gastroint Dig Syst 3:157. doi: 10.4172/2161-069X.1000157
Copyright: © 2013 Henry T Lynch MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Colorectal cancer at an early average age (approximately 44 years), with a variable spectrum of extracolonic cancers, characterizes hereditary nonpolyposis colorectal cancer/Lynch syndrome. However, even liberal criteria for the syndrome may miss a percentage of those carrying a mismatch repair mutation, suggesting the need for universal testing of all colorectal cancer patients by microsatellite instability and/ or immunohistochemistry evaluation. Such universal testing should lead to the identification of relatives of syndrome affected who would also benefit from being evaluated for the presence of the syndrome, but family dynamics as well as lack of healthcare provider interest often stand in the way. The proven benefit of appropriate screening, especially colonoscopy, calls for education of all parties as to the importance of syndrome recognition.
The U.S. Department of Health and Human Services (HHS), through its Healthy People 2020 initiative [1] has stated that “All people who are newly diagnosed with colorectal cancer should receive counseling and educational materials about genetic testing. Family members could benefit from knowing whether the colorectal cancer in their family is a hereditary form called Lynch syndrome [2]. Screening interventions could potentially reduce the risk of colorectal cancer among men and women with Lynch syndrome by 60 percent [3].” These recommendations were an endorsement of the statement by the Evaluation of Genomic Application in Practice and Prevention (EGAPP) working group [2].
One of the key recommendations of the EGAPP was the universal testing of newly diagnosed colorectal cancers (CRCs) for evidence of microsatellite instability (MSI). Whether by PCR-based methods or by immunohistochemical staining (IHC) of tumor tissue for loss of expression of proteins associated with the mismatch repair (MMR) genes, this testing was considered the key step toward diagnosis of Hereditary Nonpolyposis Colorectal Cancer (HNPCC), also known as “Lynch Syndrome” (LS). HNPCC/LS accounts for 1-4% of all CRC, [4] and is thus fairly uncommon. However, in affected families the predictability of risk is such that identification of carrier status in the index cases and at-risk relatives is seen as critical.
Our position is that universal testing of newly diagnosed CRC should be the norm. Further, that this should be linked to effective genetic counseling, not only for the cancer patient whose diagnosis triggers the evaluation, but for the extended family of potentially at-risk relatives who would otherwise not receive useful information about their risk, benefits of genetic testing, or the utility of early prevention and treatment measures.
The recommendations of Healthy People 2020 have a sound basis, beginning with evidence for the efficacy of colonoscopic screening for carriers of HNPCC/LS mutations [3,5] and extending to the utility of prophylactic hysterectomy and bilateral salpingo-oophorectomy, for reduction of endometrial and ovarian cancer risk [6]. In order for such measures to be as widely beneficial as possible, it is important that all mutation carriers in a family be identified, including not only offspring and siblings, but more distant cousins as well.
What is HNPCC/LS?
CRC at an early average age, with a variable spectrum of extracolonic tumors characterize HNPCC/LS [7-9] (Table 1). However, the advocacy for universal testing is based on two important considerations. First, even liberal criteria for HNPCC/LS frequently miss a percentage of those carrying a MMR mutation (older patients with little or no apparent family cancer history) [4]. Secondly, although our focus here is HNPCC/LS detection, oncologists are keenly interested in the prognostic and therapeutic implications of MSI, whether it occurs in the 1-4% that are HNPCC/LS, or whether it is the sporadic, nonfamilial, epigenetic event that occurs in another 10-15 % of CRCs [10,11]. In an oft-cited population series of more than 500 cases from Ohio, Hampel et al. [4] found that of the 3.6% that had HNPCC/LS, only 72% met the revised Bethesda guidelines [9], a liberal construct based on family history, age, and other factors often used to select individuals most likely to have informative microsatellite testing. In the Hampel et al. study [4], MSI and IHC were found to have sensitivities of 100% and 94.4% and specificities of 90.5% and 88.4%, respectively. The HHS, in its statement quoted above, agrees that universal testing with MSI and/or IHC should be offered.
| Autosomal dominant inheritance pattern seen for syndrome cancers in the family pedigree. |
| Earlier average age of CRC onset than in the general population: |
| average age of 45 years in Lynch syndrome vs. 63 years in the general population |
| Proximal (right-sided) colonic cancer predilection: |
| 70-85% of Lynch syndrome CRCs are proximal to the splenic flexure. |
| Accelerated carcinogenesis: (tiny adenomas can develop into carcinomas more quickly): |
| within 2-3 years in Lynch syndrome vs. 8-10 years in the general population |
| High risk of additional CRCs: |
| 25-30% of patients having surgery for a Lynch syndrome-associated CRC will have a second primary CRC within 10 years of surgical resection if the surgery was less than a subtotal colectomy |
| Increased risk for malignancy at certain extracolonic sites: |
| endometrium (40-60% lifetime risk for female mutation carriers) |
| ovary (12-15% lifetime risk for female mutation carriers) |
| stomach (higher risk in families indigenous to the Orient, reason unknown at this time) |
| small bowel |
| hepatobiliary tract |
| pancreas |
| upper uro-epithelial tract (transitional cell carcinoma of the ureter and renal pelvis) |
| prostate cancer |
| breast cancer |
| adrenal cortical carcinomas |
| brain (glioblastomas in the Turcot�s syndrome variant of the Lynch syndrome) |
| sebaceous adenomas, sebaceous carcinomas, and multiple keratoacanthomas in the Muir-Torre syndrome variant of Lynch syndrome |
| Pathology of CRCs is more often poorly differentiated, with an excess of mucoid and signet-cell features, a Crohn�s-like reaction, and a significant excess of infiltrating lymphocytes within the tumor. |
| Increased survival from CRC. |
| The sine qua non for diagnosis of LS is the identification of a germline mutation in a mismatch repair gene (most commonly MLH1, MSH2, or MSH6) that segregates in the family: i.e., members who carry the mutation show a much higher rate of syndrome-related cancers than those who do not carry the mutation. |
Table 1: Cardinal features of HNPCC/Lynch syndrome.
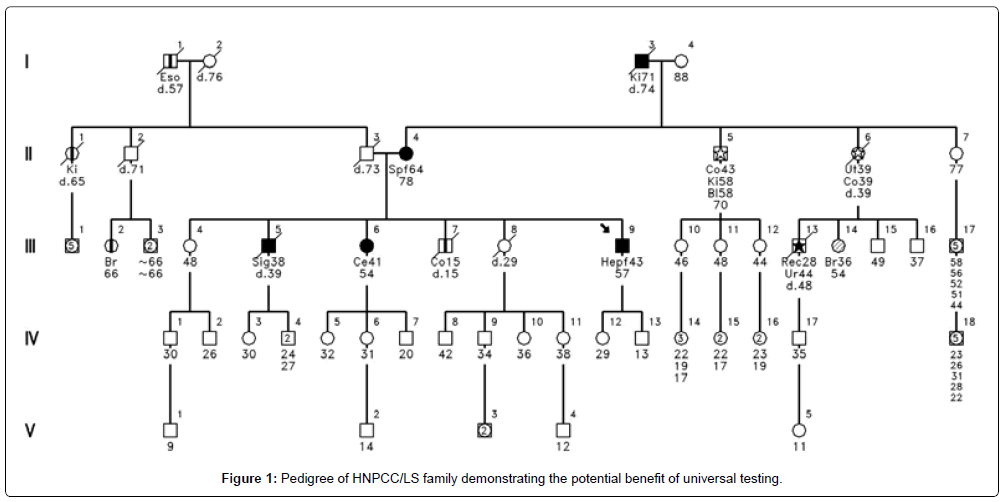
Figure 1 shows an example of the benefits of universal testing. Had universal testing been in place when patient II-4 was diagnosed with CRC at age 64, the informative test would have prompted mutational testing. His children, siblings and other relatives would have benefited from this knowledge and had a warning of their potential cancer risk. Being diagnosed at age 64 would have excluded him from current practices of restricting MSI and IHC and referral for genetic counseling to only early-onset CRC cases.
Suppose then that regular MSI testing is in place and is followed by genetic counseling and mutational testing when informative. The challenge has only begun to be engaged at the point of mutation detection in the index patient. Once a mutation is detected, carrier status, either positive or negative, can then be precisely established for any and all at-risk relatives, however near or distant they may be geographically or by degree of kinship. If widespread uptake of predictive testing were to occur in extended families, the benefits would be substantial. Several studies have found it to be cost effective to do genetic testing-driven management of risk in affected families [12-14]. Ladabaum et al., [13] noting recommendations for MSI testing for all patients with newly-diagnosed CRC, [2,4] showed through modeling analysis that such testing is, in fact cost-effective. Mvundura et al. [14] have shown comparable cost effectiveness from the perspective of the United States health care system.
Important logistic and process issues come to the fore in considering how to operationalize the testing process and outreach to families. Heald et al. [15] in recommending that all CRCs be screened through MSI and IHC, admit to uncertainty as to how this could be implemented in a health system. In their study from the Cleveland Clinic, they looked at several approaches that had been used. One approach was to send the MSI/IHC results to the colorectal surgeon. Another involved the results being received by both the surgeon and a genetic counselor, with the counselor emailing the surgeon regarding patients to be referred for genetic counseling (GC). In yet another approach, the colorectal surgeon and genetic counselor received the information but the genetic counselor contacted the patient directly to facilitate referral. MSI/IHC was found 16% of 1,108 patients. If the surgeon only was notified of results, only 55% of patients with abnormal MSI/IHC were referred for GC, with 32% undergoing GC and 26% receiving genetic testing (GT). When the genetic counselor additionally received results and prompted the surgeon, 82% were referred for GC, with 64% having GC and 45% having GT. When the counselor directly contacted patients, 100% were referred for GC, with 71% having GC and 66% receiving GT. Time from referral to GC was 10-fold quicker in this last approach. The authors concluded that MSI/ IHC with GC/GT, when implemented with effective multidisciplinary communication and proactive patient contact, led to increased identification of patients with HNPCC/LS.
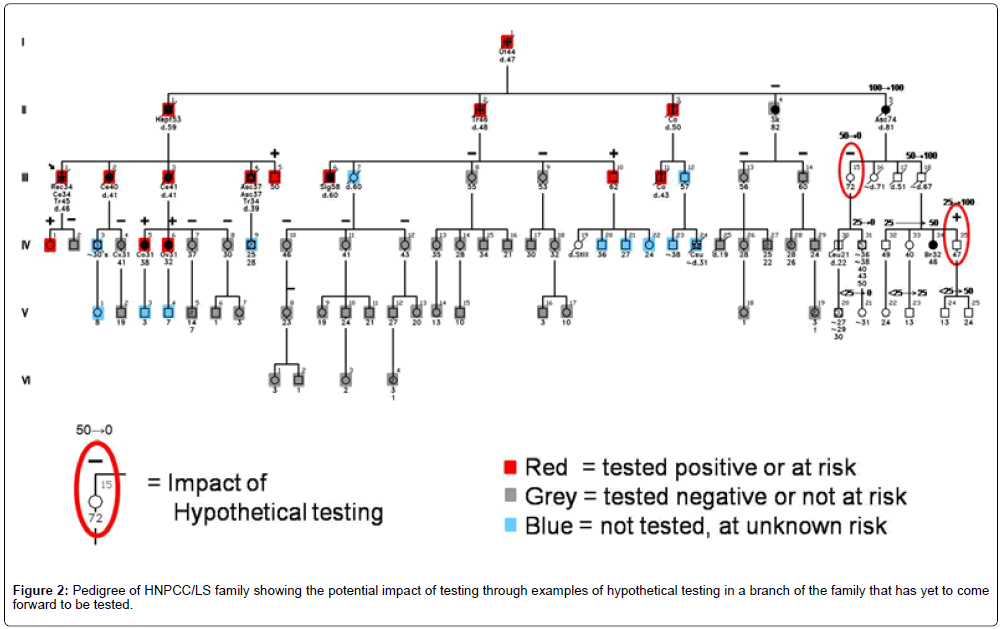
Do we really know how genetic testing plays out in actual families? The question of how genetic testing can alter the cancer risk status of other family members was partially answered by Watson et al. [16]. They studied Creighton’s HNPCC/LS and hereditary breastovarian cancer (HBOC) syndrome families with known mutations, demonstrating, as predicted, that testing in relatives reclassified risk status from uncertain to certain (from 50% risk to either carrier or non-carrier status). In 60% of cases, carrier status could be reassigned based on a relative’s test result. Figure 2 depicts the testing impact on a family through examples of hypothetical testing in a branch of the family that has yet to come forward to be tested. These status changes fundamentally alter the recommended surveillance and prevention practices for individuals whose risk is reclassified. Mutation carriers warrant aggressive surveillance, while non-carriers can safely follow general population screening guidelines.
However, uptake of predictive testing has been disappointing in first-degree relatives of index patients, and has been even more limited in second- and more distant-degree relatives. A possible method to increase uptake of testing and genetic counseling is the Family Information Service (FIS), which involves an in-person session in which expert providers and counselors meet with multiple family members in a convenient geographical location [17,18]. Education and counseling are intended to lead to testing for the family deleterious mutation, followed by appropriate surveillance.
A study was done targeting 97 Lynch syndrome families with a known deleterious mutation [19]. Selection for FIS was based on family size and convenient geographic location. Twenty-eight families were offered an FIS and 69 received standard care (mailed educational material and invitation for testing). Data were collected on testing rates. Among families that received an FIS, 20.4% of the at-risk relatives were DNA tested, whereas in families that did not receive an FIS, 12.9% were DNA tested. The difference in proportions tested between the FIS and non-FIS families was statistically significant (p=0.003) and was more pronounced in family members whose relationship to the proband was beyond first-degree (p<0.0001). Of those individuals who attended an FIS, 81.1% were tested. It was concluded that genetic counseling in the FIS setting facilitates uptake of predictive mutational testing in relatives of the index case. However, the FIS is time-consuming and labor intensive; more efficient means of disseminating information on hereditary cancer risk and DNA testing to relatives are needed.
Conclusion
We anticipate that MSI/IHC testing of newly diagnosed CRCs will become universal in years to come, unless the cost and sensitivity of direct germline MMR gene testing improve significantly. It will be incumbent on clinicians to appreciate the meaning of the MSI/IHC test results and to integrate genetic counselors into the process of translating information test results into efficient genetic counseling prior to germline mutational testing. Once mutations are detected, modern, preferably technology-driven communications within families will need to occur if those potentially benefitting from predictive testing are to actually receive such testing. It must be kept in mind that there remains a subset of families with the HNPCC/LS phenotype in which current testing methods have not been able to identify a germline MMR mutation. Until we are able to pinpoint the mutation responsible for HNPCC/LS in all cancer patients that are thought to have the condition, they and their at-risk relatives will need to undergo surveillance “as if ” HNPCC/LS carrier status had been established. Because this is a more difficult concept to grasp than even the situation in mutation/known families, mutation-negative families will need to have greater attention devoted to their counseling and surveillance needs.
References
- Healthy People 2020 Topics and Objectives (2013) Genomics. U S Department of Health and Human Services.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009) Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 11: 35-41.
- Stupart DA, Goldberg PA, Algar U, Ramesar R (2009) Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis 11: 126-130.
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, et al. (2008) Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 26: 5783-5788.
- Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, et al. (2000) Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118: 829-834.
- Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, et al. (2006) Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 354: 261-269.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT (1991) The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 34: 424-425.
- Vasen HFA, Watson P, Mecklin J-P, Lynch HT(1999) ICG-HNPCC. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 116: 1453-1456.
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96: 261-268.
- Sinicrope F, Foster NR, Sargent DJ, Thibodeau SN, Smyrk TC, et al. (2010) North Central Cancer Treatment Group. Model-based prediction of defective DNA mismatch repair using clinicopathological variables in sporadic colon cancer patients. Cancer 116: 1691-1698.
- Sinicrope FA, Rego RL, Garrity-Park MM, Foster NR, Sargent DJ, et al. (2007) Alterations in cell proliferation and apoptosis in colon cancers with microsatellite instability. Int J Cancer 120: 1232-1238.
- Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, et al. (2011) Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 4: 9-22.
- Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, et al. (2011) Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 155: 69-79.
- Mvundura M, Grosse SD, Hampel H, Palomaki GE (2010) The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 12: 93-104.
- Heald B, Plesec T, Liu X, Pai R, Patil D, et al. (2013) Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing Lynch syndrome in a large acadmic medical center. J Clin Oncol 31: 1336-1340.
- Watson P, Narod SA, Fodde R, Wagner A, Lynch JF, et al. (2003) Carrier risk status changes resulting from mutation testing in hereditary non-polyposis colorectal cancer and hereditary breast-ovarian cancer. J Med Genet 40: 591-596.
- Lynch HT (2001) Family information service and hereditary cancer. Cancer 91: 625-628.
- Lynch HT, Snyder CL, Lynch JF, Ghate S, Narod SA, et al. (2009) Family information service participation increases the rates of mutation testing among members of families with BRCA1/2 mutations. Breast J 15 Suppl 1: S20-24.
- Lynch HT, Buxbaum S, Snyder CL, Stacey M, Shaw T, et al. (2013). The impact of family information services on genetic testing uptake among relatives in Lynch syndrome families. Presented 3 June 2013 at ASCO Annual Meeting, Chicago, IL (Abstract).
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13774
- [From(publication date):
December-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9243
- PDF downloads : 4531