Review Article Open Access
Wastewater Treatment by Combination of Advanced Oxidation Processes and Conventional Biological Systems
| Alessandra Cesaro*, Vincenzo Naddeo and Vincenzo Belgiorno | |
| SEED - Sanitary Environmental Engineering Division, Department of Civil Engineering, University of Salerno via Giovanni Paolo II, 84084 - Fisciano (SA), Italy | |
| Corresponding Author : | A Cesaro SEED - Sanitary Environmental Engineering Division Department of Civil Engineering University of Salerno via Giovanni Paolo II 84084 - Fisciano (SA), Italy E-mail: acesaro@unisa.it |
| Received: August 30, 2013; Accepted: September 30, 2013; Published: October 05, 2013 | |
| Citation: Cesaro A, Naddeo V, Belgiorno V (2013) Wastewater Treatment by Combination of Advanced Oxidation Processes and Conventional Biological Systems. J Bioremed Biodeg 4:208. doi:10.4172/2155-6199.1000208 | |
| Copyright: © 2013 Cesaro A, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
One of the most challenging issues of the last decades is the presence of recalcitrant compounds in the effluents of wastewater treatment plants, due to their toxicity on both human health and environment. Although conventional biological processes are usually efficient for the degradation of pollutants occurring in wastewaters, most of these compounds are not effectively removed.
In this context, Advanced Oxidation Processes (AOPs), which are oxidation methods relying on the action of highly reactive species such as hydroxyl radicals, are raising great interest for the removal of those organic pollutants not treatable by conventional techniques due to their high chemical stability and/or low biodegradability. As several studies pointed out the effectiveness of AOPs in the degradation of a wide spectrum of both organic and inorganic pollutants, they are considered a highly competitive wastewater treatment technology. However, in order to reduce operating costs associated to the application of AOPs, their proper combination with conventional biological processes should be considered.
This work aims to discuss the most common AOPs used as pretreatment of wastewater for its biological processing, in order to highlight the enhancement of wastewater biological treatability supplied by different advanced oxidation methods. To this end, main standard tests and parameters for wastewater biodegradability assessment are also pointed out, thus providing an overview of the most reliable ones.
| Keywords |
| Biodegradability; Emerging contaminants; Fenton processes; Free radicals; Oxidation; Ozone; Photocatalysis; Recalcitrant compounds; Ultrasound |
| Introduction |
| In the last years, one of the major concerns to water quality is related to the detection of chemical pollutants in both industrial and municipal wastewater. Most of these contaminants, both synthetic organic chemicals and naturally occurring substances, enter the aquatic medium in several different ways and, according to their watersolubility, can be transported and distributed in the water cycle [1]. |
| The risk associated to these contaminants, such as pharmaceuticals, endocrine disruptor, personal care products, pesticides, is related to their ubiquity and persistence into the environment as well as to their biological activity that may affect the development of aquatic organisms and wildlife [2]. |
| The effluents of urban wastewater treatment plants are among the major responsible for the release of this kind of contaminants into the environment [2,3]. Although conventional biological processes are usually efficient for the degradation of pollutants occurring in wastewater, refractory compounds are not effectively removed [4]. |
| In such cases the use of Advanced Oxidation Processes (AOPs) may improve the overall removal efficiency of such compounds. |
| AOPs are based on the chemistry of hydroxyl radicals (• OH), which are non-selective reactive species, able to oxidize pollutants into mineral end-products, yielding CO2 and inorganic ions [5]. |
| However, the use of AOPs is not cost-effective if intended to mineralize toxic and recalcitrant compounds in wastewater [1]. Therefore, suitable application of AOPs should not consider, whenever possible, the replacement of the more economic biological processes [6], but the proper combination of both systems. |
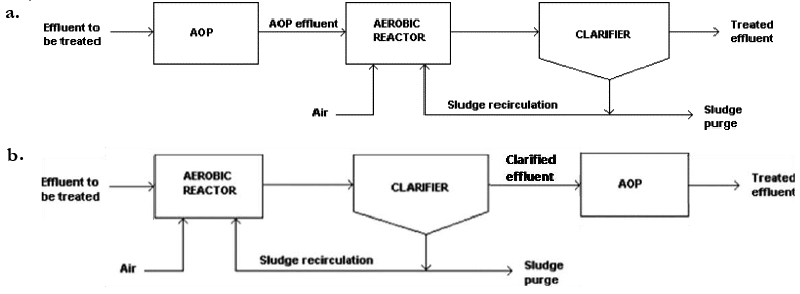
| AOPs can be used as pre- and/or post-treatment of biological systems (Figure 1). In the former case, AOPs aim to improve biological treatability of wastewaters, thus favouring their processing by means of common microorganisms [7-9]. In the latter, the oxidation step is directed towards the removal of those contaminants not completely degraded during the biological treatment [10]. |
| In order to ensure the economic optimization of the combined process, it is necessary to limit the intensity and/or duration of the advanced treatment. As a result, special attention must be paid to the procedures useful to evaluate the efficiency of the process. When AOPs are used as pretreatment of wastewater for their biological processing, their performances have to be adequately assessed through biodegradability tests [11]. |
| This work discusses the most studied AOPs used as pretreatment of wastewaters for biological processing, in order to highlight the enhancement of wastewater biological treatability supplied by different advanced processes. To this end, wastewater biodegradability assessment is pointed out, with reference to the most spread standard tests and parameters, thus providing an overview of the most reliable ones. |
| Degradation Mechanisms by Advanced Oxidation Processes |
| The efficacy of AOPs in improving biological degradability of recalcitrant compounds in wastewater depends on both chemical and physical properties of contaminants as well as on the generation of reactive free radicals, in most cases hydroxyl radicals [12]. The oxidation reaction between these radicals and the contaminants is the mechanism behind the degradation of the contaminant itself. |
| The generation of these reactive agents can be achieved by means of several processes, including, sonolysis [13], ozone-based processes [14], Fenton-based reactions [15], heterogeneous photocatalysis [16] and various combination of these technologies [17-19]. Each one can be characterized according to the specific method for the production of free radicals. |
| Sonochemical processes imply the application of ultrasound (US), which refers to sound waves with a frequency ranging between 20 kHz and 500 MHz. When ultrasound propagates in a liquid, it promotes the formation of cavitational bubbles, whose collapse is associated to both physical and chemical effects [13]. In particular, at high frequencies, chemical ultrasonic effects are predominant due to the larger formation of free radicals [20]. |
| These radicals move to the liquid-gas interface to react with the organic substrate [21] or, in the case of high concentration, they recombine with each other to form H2O2 [22], which is an oxidative agent as well, thus providing the degradation of contaminants. |
| Sonolysis is a versatile process, which has been widely studied for the degradation of several compounds [23-25] even in combination with other AOPs [26]. Its main disadvantage is related to energy consumption. This item often limits the applicability of the ultrasonic technology to small volumes. Differently, ozonation has shown a very strong oxidizing power with short reaction times, thus allowing the treatment of great amount of wastewaters. |
| The process relies on ozone, which is unstable in an aqueous medium. It decomposes spontaneously by a complex mechanism that involves the generation of hydroxyl free radicals. Therefore, the degradation of pollutants occurs by both ozone itself and radicals [27], although the latter is more powerful than the former, as highlighted in Table 1, reporting the reaction rate constants for both oxidants with reference to several compounds. |
| Although ozonation has already been applied at full-scale, as already pointed out for sonolysis, it is an energy intensive process, characterized by high operating costs, mainly associated to ozone generation. |
| As ozone is an unstable molecule, it should be generated at the point of application. To this end, several methods can be used, but the most common within ozone generation industry is the corona discharge one, which requires a considerable energy input. |
| Ozone technology has also been studied in combination with ultraviolet (UV) radiation, since UV photons are able to activate ozone molecules. In this way, the formation of hydroxyl radicals is promoted [28,29], but any relevant energy saving can be pursued. |
| UV radiation, in the wavelength range between 200 and 280 nm, can also be applied in combination with hydrogen peroxide (H2O2). The major drawback of this process is related to the small molar extinction coefficient of H2O2. Therefore, only a relative small fraction of incident light is exploited, especially when organic substrates will act as inner filters. Moreover, the rate of photolysis of aqueous H2O2 is pH dependent: it was found to increase when more alkaline conditions are used [6]. |
| H2O2 occurs also in Fenton based processes: its reaction with iron in water, under acidic conditions, determines the formation of radicals. The rate constant for the reaction of ferrous ion with hydrogen peroxide is high and Fe(II) oxidizes to Fe(III) in a few seconds to minutes in the presence of excess amounts of hydrogen peroxide, which decomposes by Fe(III) and generates again hydroxyl radicals. The major parameter affecting Fenton processes are: the pH of the solution, the amount of ferrous ions, the concentration of H2O2, the initial concentration of contaminants and the presence of other ions [30]. Moreover, Fenton reagent action can be significantly improved when exposed to UV radiation [31]. |
| Enhancement of reagent yields after light irradiation is the concept on which also photocatalytic processes have been developed. |
| Heterogeneous photocatalysis is a photochemical reaction, accelerated by the action of a catalyst: one of the most widely used and highly effective is TiO2 [32]. The mechanism action is based on the transition of electrons from the valence to the conduction band, which is caused by the light irradiation of the catalyst. In particular, both migrating electrons and the holes created in the valence band can participate in redox reactions with compounds absorbed on the photocatalyst [33]. The presence of molecules which compete with the contaminants for reactive sites should be, therefore, avoided. |
| Notwithstanding the possibility of mineralization of several compounds, photocatalysis full-scale application is still not spread due to both technical and economic reasons, mainly related to the proper handling of the catalyst. |
| Table 2 summarizes main advantages and drawbacks for the discussed AOPs. |
| The design of AOPs depends on several parameters, including: reagent dosage and ratios with other substances, contact time and reactor configuration. The optimal conditions have to be determined with reference to the treatment scenario of interest [34]. Reasonably, longer contact time as well as higher reagent dosage result in more effective treatment but also in operating costs which can be not sustainable. |
| Differently, when AOPs are used in combination with conventional biological treatment of wastewaters, their application is not intended to remove refractory compounds and can be cost-competitive. In this contest, the feasibility of AOPs is highly dependent on the enhancement of wastewater biological treatability and, consequently, the assessment of biodegradability plays a fundamental role. |
| Wastewater Biodegradability Assessment |
| In scientific literature, biodegradability concept has been used to refer to different characteristics of a substrate, such as persistence [35] or bioavailability [36]. In the field of water and wastewater treatment, biodegradability often implies the biological treatability of the investigated substrates [37]. |
| Due to these differences, several tests have been developed in time to assess biodegradability. |
| OECD (Organization for Economic Cooperation and Development) guidelines distinguish three main groups within the biodegradability test system [38]: |
| - Ready Biodegradability Tests (RBTs), which are useful for quick screening. They all rely on the principle that biodegradation is monitored as the degree of mineralization, by means of aggregated parameters such as oxygen uptake, carbon dioxide production or reduction of dissolved organic carbon (DOC); |
| - Inherent Biodegradability Tests (IBTs), to demonstrate the potential degradability of a compound. Differently from RBTs, biodegradation conditions are optimized, thus making them really reliable; |
| - Simulation Tests (STs), designed to measure the rate of biodegradation in a specified environmental compartment. Test substance concentration varies according to the test aim: it is lower, if intended to provide biodegradation rates; higher to quantify main degradation products. The measurement of degradation rates, moreover, requires specific analysis. |
| According to this classification, a fundamental step for the evaluation of wastewater biodegradability is the performance of RBTs. A compound can be considered readily biodegradable, if the results of RBTs fit the following criteria [39]: |
| - O2 uptake or CO2 evolution achieves at least 60% of the theoretical one or DOC removal reaches 70%; |
| - time elapsed from the start of the mineralization process, defined as 10% of the theoretical one until the required plateau is reached, should be no longer than 10 days. |
| According to OECD guidelines, if these conditions are not fulfilled, the test substance cannot be considered "not biodegradable", but should undergo additional trials, even within the class of the RBTs. Although some of these tests based on respirometry for the determination of O2 uptake are more versatile than others, their applicability depends also on the kind of substances which are being investigated, as shown in Table 3 [40]. |
| Table 4 lists the most performed IBTs, highlighting that the population density is higher than the one of RBTs. This item makes the conditions for the biodegradation optimal. Therefore, a negative result would indicate a high persistence of the test substance, suggesting that no further research on biodegradation should be performed [38]. |
| An important aspect to be taken into account is reproducibility of test results. |
| One of the most recent studies on the topic [11] was carried out comparing different tests to determine the biodegradability enhancement during the advanced treatment of wastewater samples containing 200 mgDOC/L of a pesticide mixture. Authors found that the results of Zahn-Wellens test were consistent with the ones achieved through the Pseudomonas putida bioassay. The use of this bacteria is standardized within the procedures provided by DIN 38 412 Part 8 (1991) and DIN 38 412 Part 27 (1993) to assess water and wastewater toxicity, by evaluating the growth inhibition in 30 minutes. In the study of Ballesteros Martín et al. [11], the same bacteria species was used as culture mean for a bioassay, incubated for 120 h. As for the Zahn- Wellens tests, biodegradability efficiency of the investigated AOP was assessed in terms of DOC removal. |
| Results showed that both Zahn-Wellens test and Pseudomonas putida bioassay proved to be the most suitably judging by repeatability and precision. The main advantage of the Pseudomonas putida test is the shorter time required to obtain reliable results, in comparison to the Zahn-Wellens test, lasting 28 days. |
| The duration of biodegradability tests can be a discriminating factor in the choice of the test itself as well as the operating simplicity, especially in research screening steps. This item has promoted the use of BOD5/COD ratio, which is quite spread in literature [41] as biodegradability indicator: when the ratio is higher than 0.4, the test substance is considered biodegradable [42]. |
| Although biodegradability tests provide useful information concerning the effect of chemical pre-treatment on subsequent biological degradation of wastewater, experiments integrating chemical and biological degradation are necessary for a more realistic viewpoint of the combined process [1]. |
| Combined AOPs and Biological Processes for Wastewater Treatment |
| Most studies dealing with AOPs as pretreatment of wastewater for their biological processing refer to laboratory and pilot scale tests. |
| One of the main obstacles to the scale up of AOPs for the treatment of wastewater prior to biological processes is related to the oxidant dose. High reagent concentrations determine significant increases in operating costs as well as serious damages to microorganisms [43,44]. On the other hand, low reagent doses could result in inadequate pretreatment of wastewaters. |
| The effectiveness of AOPs has been extensively proved for the pretreatment of several kinds of wastewaters, including industrial ones [45], as they can be conveniently reused within the productive process. |
| According to Scottis and Ollis [46], the kinds of wastewater that can be successfully treated by means of combined AOPs/biological processes are the ones containing bio-resistant or recalcitrant compounds, which are often of industrial origin, as well as the wastewaters containing pollutants resulting in toxicity for microorganisms. |
| Among bio-resistant compounds, pesticides arise great concern, since their high solubility makes their propagation in the environment extremely easy. Although several processes have been studied for the pretreatment of wastewater polluted by pesticides, the most recent trend is directed toward the combination of Fenton and photo-Fenton processes with aerobic biological treatment [47,48]. |
| Zapata et al. [49] found that the photo-Fenton treatment at pilot plant scale was able to increase the biodegradability of a wastewater polluted with commercial pesticides from 50% to 95% as well as to reduce its toxicity (from 96% to 50% of inhibition). Authors also observed that the most suitable point for combining the photo-Fenton process with the biological treatment was after the total elimination of the active ingredients. The efficiency of the combined photo-Fenton/ biological system in terms of mineralization was 94%, while the combination bio/photo-Fenton was not successful, thus pointing out the importance of the proper identification of the sequence within treatment units. |
| Fenton based processes have been also applied to several industrial wastewaters, such as tannery effluents, which are usually characterized by low pH, relatively high temperature and high presence of aromatic compounds. |
| In the study of Mandal et al. [50], the application of Fenton process as pretreatment for a biological system allowed the reduction of pollutant content, in terms of both COD and BOD5, thus improving the biodegradability and reducing the duration of biological treatment. The main drawback of the combined Fenton/biological process was the high production of sludge (about 3 kg dry sludge/m3), which greatly affect the economic balance, as observed also in the study of Di Iaconi et al. [51]. |
| Fenton raection was also studied by Feng et al. [52] in combination with a membrane bioreactor (MBR) for the advanced processing of the effluent from an integrated dyeing wastewater treatment plant. In this study, Zahn-Wallens Test was used to assess the wastewater biodegradability enhancement after Fenton process. However, the same effect was also evaluated in terms of TOC, after the Fenton treatment as well as after the combined Fenton/MBR system. Although a IBTs was performed, the estimation of TOC allowed a prompt comparative assessment between the single AOP and its combination with a biological system by means of a parameter that is common and easy to determine. |
| Similar consideration arises for the study of Oller et al. [53], reporting the combination, at pilot scale, of Fenton process with an attached biomass biological reactor, for the treatment of 4 m3/d of pharmaceutical wastewater, with a concentration of 600 mg/L of α-methylphenylglycine and a DOC value in the range 400-600 mg/L. In this case, the removal reached through the combined process was evaluated in terms of DOC and was found to reach values up to 95%. |
| Pharmaceuticals represent only one of the widest categories of concern among emerging contaminants because of their endocrinedisrupting properties. Personal-care products, steroid sex hormones, illicit drugs, flame retardants and perfluorinated compounds are other particularly relevant examples of such emerging compounds, whose high transformation/removal rates are compensated by their continuous introduction into the environment. In order to increase biodegradability and detoxify effluent streams containing such compounds, alternative treatments with AOPs have been studied [54-56]. |
| Naddeo et al. [24] investigated the application of sonolysis on the degradation of three kinds of pharmaceuticals, both in single solutions and as mixtures spiked in urban wastewater effluent. Several operating conditions were studied and the aerobic biodegradability variation assessed by BOD5/COD ratio. It was found that the pharmaceuticals conversion enhanced for increasing ultrasonic power densities. Reaction by-products proved to be more stable than the original compounds as well as more readily biodegradable, thus suggesting the effectiveness of sonolysis as pretreatment rather than post-treatment. In the former case, lower energy input can be provided in order to achieve an adequate increase in biodegradability and promote the consequent processing of wastewater by conventional biological systems. |
| The great potential of ultrasonic irradiation for the degradation of toxic organic compounds in wastewater was also highlighted in the study of De Bel et al. [57]. Authors found that, although there was only a minor decrease in COD after treatment, the BOD/COD ratio of the antibiotic solution increased from 0.06 to a maximum of 0.60. |
| Sonolysis has been widely investigated as wastewater treatment prior to biological processes [58,59] for the increase of different organic substrates biodegradability [60-62], also in combination with other AOPs [63]. Most studies, however, are focused on the effects of AOPs on organic substance removal rather than the improvement in biodegradability. Sangave et al. [64] evaluated the effectiveness of a combined US/ozone process in improving the aerobic degradation of distillery wastewater and observed a COD reduction up to 45%. |
| A more recent work was carried out with reference to ozonation, applied in the treatment line for remediation of different kinds of wastewater. Integrated schemes considering ozonation alone as both pre- and post-treatment for the biological processing of distillery wastewater allowed around 79% reduction of pollutants, expressed as COD, compared to 35% COD reduction with a not ozonated sample [65]. |
| Similarly, Di Iaconi et al. [66] operated at demonstrative scale an aerobic granular biomass system (SBBGR – Sequencing Batch Biofilter Granular Reactor) integrated with ozonation for the treatment of tannery wastewater. Results showed the removal efficiencies of the combined process for several parameters, including COD, TSS, TKN, as well as the estimation of sludge production for the assessment of the process economic feasibility. The same process scheme was used in the study of Lotito et al. [67] for the treatment of textile wastewater. |
| In all cases, any test to assess changes in biodegradability after ozone application was performed, whereas several studies dealing with the assessment of biodegradability enhancement after the application of ozonation to specific kinds of wastewaters are reported in literature (Table 5). |
| As shown in Table 5, BOD5/COD ratio was found to be the most common parameter used to assess the biodegradability of a test substance after ozonation, even in combination with other AOPs [68-75]. |
| The recurring use of this parameter is related to the operational simplicity, although several tests have been standardized to assess biodegradability and to provide specific information about this property. |
| Conclusion |
| Advanced Oxidation Processes represent one of the most promising options for the removal of persistent compounds in wastewater treatment effluents. |
| The action mechanism of AOPs relies on the formation of high reactive oxidant species, mainly hydroxyl radicals, which can react with recalcitrant compounds until their mineralization occurs. However, when AOPs are intended to remove all these pollutants from wastewaters, their application can be not sustainable. Conversely, their combination with conventional biological processes can be considered a valid option. It has been extensively proved that AOPs can improve the biological treatability of wastewaters, thus enhancing the removal of both organic matter and recalcitrant compounds. |
| In this contest, the assessment of biodegradability variation after the application of AOPs plays a fundamental role, so that specific procedures have been standardized in time. However, even though they are now well developed, results of the biodegradability variation after AOPs are usually expressed in terms of BOD5, COD, BOD5/COD ratio, DOC. Differently from biodegradability assays, these parameters are easy and quick to determine. Moreover, their recurring occurrence in scientific literature allows the immediate comparison of results obtained from different studies dealing with the use of AOPs as wastewater pretreatment for its biological processing. |
| This aspect is particularly important when considering that the investigation of advanced treatment effects generally follows two different approaches. |
| In the first one, research is focused on the effectiveness of AOPs in improving wastewater biodegradability. This approach is developed to deepen the study of the viability of the investigated AOPs as biological system pretreatment and/or to assess the qualitative characterization of its intermediates. |
| In the second one, aim of the experimental study is the integrated AOPs/biological process feasibility. This second kind of approach is pursuable when the enhancement of biodegradability after the application of the studied AOPs is already clear and the feasibility of the combined process has to be assessed. |
| Therefore, the comprehension of the action mechanisms of investigated AOPs has been extensively studied and the potential of several processes has been recognized. |
| The gap that scientific research should cover is the assessment of the technical and economic feasibility of AOPs as treatment of wastewater before its conventional biological processing. To this end, further research should be mainly addressed towards: |
| - the definition of removal kinetics of pollutants after combined AOPs/biological processes, in order to optimize the operating conditions as well as to identify modelling tools to generalize experimental data; |
| - The assessment of the combined AOPs/biological process efficiency in larger scale continuously operated systems, in order to promote its scale up. |
| Acknowledgements |
| Research activities were partly funded by the FARB project of the University of Salerno and PRIN project founded by Italian Ministry of University. Assistance provided by P. Napodano is deeply appreciated. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 18092
- [From(publication date):
September-2013 - Jul 03, 2024] - Breakdown by view type
- HTML page views : 13040
- PDF downloads : 5052
