Vocal Cord Dysfunction:An Updated Review
Received: 02-Nov-2011 / Accepted Date: 04-Dec-2011 / Published Date: 08-Dec-2011 DOI: 10.4172/2161-119X.S1-002
256956Introduction
Vocal cord dysfunction (VCD), also referred to as paradoxical vocal cord motion, describes a functional disorder of the larynx characterized by abnormal vocal cord motion in the absence of organic pathology. It manifests most commonly as a paradoxical and inappropriate adduction of the vocal cords occurring during inspiration. The obstruction to airflow through a narrowed glottic opening results in dyspnea and stridor. First described by Dunglison [1] in 1842, this clinical entity has been characterized as distinct syndromes using a wide variety of names. These include Munchausen stridor [2], hysterical stridor [3], functional laryngeal stridor [4], pseudoasthma [5], factitious asthma [6], emotional laryngeal wheezing [7], psychogenic upper airway obstruction [8], episodic laryngeal dyskinesia [9], episodic paroxysmal laryngospasm [10] and irritable larynx syndrome [11]. The term “vocal cord dysfunction” was first used by Christopher in 1983 [12] and “paradoxical vocal fold dysfunction” by Patel in 2004 [13]. The objective of this review is to achieve a clear understanding of the etiology, pathophysiology, clinical manifestations, diagnosis, differential diagnoses and management of VCD.
Epidemiology
The medical literature concerning VCD consists mostly of sporadic case reports and retrospective studies. Because of the lack of prospective studies based on large groups of population, the overall prevalence of VCD is unknown. It is generally believed to be rather uncommon. In certain special subgroups, however, VCD is more prevalent. Thus, VCD often co-exists with asthma. In patients with refractory asthma, 10% of adults [14] and 14% of children [15] were found to have VCD. Similarly, VCD was diagnosed in 22% of patients with a history of frequent visits to the emergency room for recurrent attacks of dyspnea [16]. In a group of military recruits with exertional dyspnea, 15% were found to have this disorder [17]. In a study of elite athletes, the prevalence of inspiratory stridor was found to be 5% [18]. Some studies have linked VCD to obesity, severe gastroesophageal reflux disease and chronic allergic rhinitis. In early reports, VCD was found to occur most often in women between 20-40 years of age, many of them health care workers [19]. The female/male ratio was 3:1 [20]. In more recent studies, however, VCD has been reported in all age groups including adolescents, children and even infants [21].
Pathogenesis and Predisposing Factors
The pathophysiology of VCD remains incompletely elucidated. Different studies often present different findings and suggest conflicting theories. Thus, early reports associated VCD with various psychosomatic or psychiatric illnesses such as anxiety disorders, depression, history of sexual abuse and post-traumatic stress syndrome. One study found that as much as 73% of patients with VCD had a psychiatric diagnosis [19]. In highly competitive young female athletes and young military recruits, VCD has been reported to be caused by exercise [22], particularly exercise related to high stress athletic competitions [23]. More recent studies, however, have failed to confirm the higher incidence of a psychiatric background in VCD patients as compared to the general population [15]. In contrast to the prior emphasis on psychopathology as the main underlying causative factor, current studies shift the focus on the association between VCD and various irritants that may trigger the glottic closure reflex.
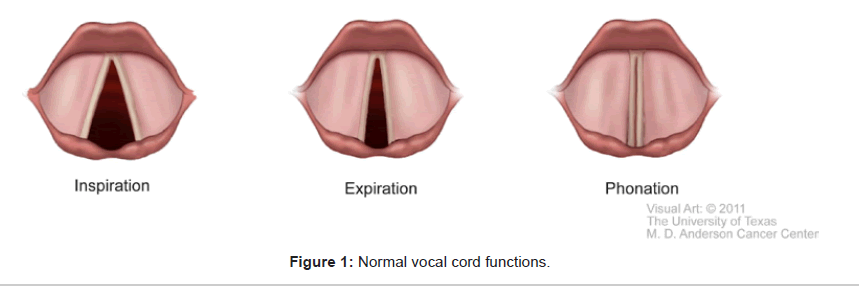
The larynx serves vital functions of breathing, phonation, and airway protection. During normal inspiration, under the control of the respiratory center in the medulla via the vagus nerve, the posterior cricoarytenoid muscle (PCA) contracts to abduct the vocal cords to enlarge the glottic opening. During expiration, the relaxation of the PCA coupled with the contraction of the lateral cricoarytenoid muscle results in a 10-40% reduction of glottic opening [24]. Thus, the vocal cords normally partially adduct during the early phase of expiration. Normal vocal cord adduction also occurs during swallowing, phonation, the Valsalva maneuver and the early compressive phase of coughing. These normal vocal cord functions are illustrated in Figure 1.
The larynx receives very extensive sensory and motor innervation. With repeated stimulation and excitation by noxious intrinsic and extrinsic irritants, these nerve fibers may become hyperexcitable and hyperresponsive. Consequently, the threshold for activation of the reflex responsible for vocal cords closure is lowered. The underlying pathophysiology of VCD involves a hyperfunctional and inappropriate laryngeal closure reflex [25]. Long-term laryngeal irritation and recurrent triggering of the glottic closure reflex can ultimately lead to an altered state of the autonomic nervous system balance [26] and contribute to reinforce and perpetuate laryngeal hypersensitivity. Laryngeal hyperresponsiveness is physiologically analogous to bronchial hypersensitivity in asthma and nasal hyperreactivity in allergic rhinitis. Endogenous or exogenous laryngeal irritants can trigger abnormal vocal cord adduction and dyspnea attacks. Thus, chronic post-nasal drip secondary to allergic rhinitis and chronic sinusitis may ultimately lead to vocal cord hypersensitivity [27].
Similarly, chronic untreated gastroesophageal reflux disease may result in the reflux of acid gastric contents into the pharynx and larynx, causing irritation and chronic inflammation of the cords leading to vocal cord hypersensitivity [28]. Finally, inhaled allergens and various airborne irritants such as smoke, dust, fumes, perfumes, organic solvents, cleaning chemicals, and ammonia have been linked to VCD [29]. Isolated reports have also described VCD occurring after exposure to glutaraldehyde [30] and after swimming in a pool containing chlorine [31].
VCD is a complex clinical entity resulting from multifactorial psychological and organic etiologies. Each causative factor may act separately or in association with others, depending on each particular situation.
Clinical Signs and Symptoms
Patients with VCD typically present with episodic and recurrent attacks of dyspnea. The distressing sensation of having to struggle to take a breath constitutes the predominant complaint. The severity of respiratory distress is quite variable, reflecting the degree of airway obstruction. Often, dyspnea is relatively mild. In other cases, it can be very severe. It often occurs suddenly without warning signs. Dyspnea is accompanied by high-pitched stridor heard loudest over the larynx and upper trachea. A choking sensation, cough, throat and chest tightness and dysphonia are also common symptoms. Typically, these symptoms do not respond to standard asthma therapy such as bronchodilators and corticosteroids. Fortunately, such attacks are usually self- limiting and subside spontaneously in less than two minutes. In exerciseinduced VCD, dyspnea typically improves rapidly with cessation of exercise. In between recurrent attacks, patients are usually completely asymptomatic, except in a few patients who may experience minor residual voice changes. While the vast majority of VCD cases are self- limiting, one atypical case has been reported in which stridor persisted for over several weeks [32]. Another case report described severe resistant VCD in a patient whose failure to respond to all available therapeutic interventions ultimately resulted in a permanent tracheostomy [33]. There have been no reports describing death or residual disability from long-term cerebral hypoxia related to VCD.
Investigation and Diagnosis
Routine laboratory tests are usually not very helpful. Only severe attacks of VCD are associated with arterial blood gases that show hypoxia with desaturation [34]. Severe anxiety secondary to difficulty breathing during attacks may also lead to hyperventilation and acute respiratory alkalosis with symptoms of lightheadedness and circumoral paresthesias. These findings are common to both VCD and asthma. Chest radiographs obtained during acute attacks usually do not demonstrate any abnormalities, in contrast to severe asthma which may show lung hyperinflation and peribronchial thickening [35].
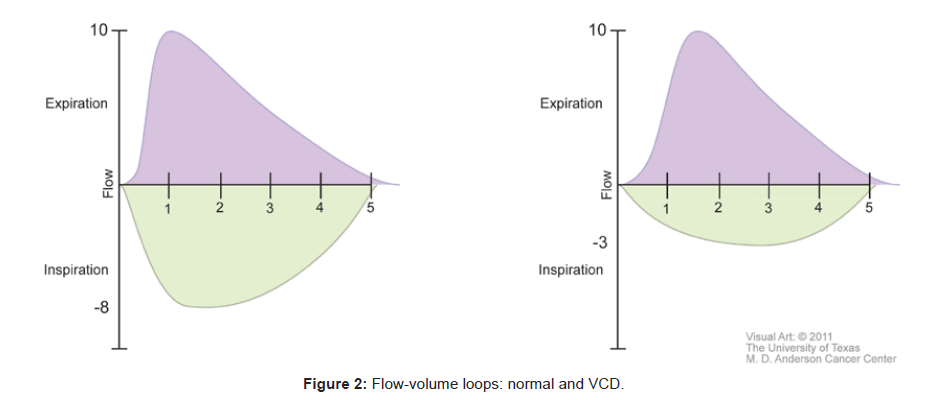
Spirometry, pulmonary function tests and flow volume loops have been used to support the diagnosis of VCD. During acute attacks, with paradoxical adduction occurring during inspiration, the maximal inspiratory flow rate decreases while the maximal expiratory flow rate does not change. Flow-volume loops demonstrate a significant flattening of the inspiratory curve, while the expiratory curve remains unchanged during acute attacks [15]. A normal flow-volume loop is shown next to one affected by VCD in Figure 2.
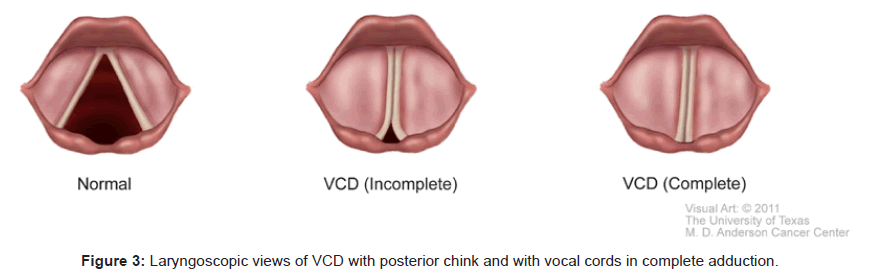
Videolaryngostroboscopy directly visualizes and documents the vocal cords adducting paradoxically during inspiration. This constitutes unquestionably the gold standard to confirm the diagnosis of VCD, especially if a posterior chink [15] is seen during an attack of stridor. Figure 3 shows laryngoscopic findings of VCD. In one exceptional report, diagnostic laryngoscopic findings confirming VCD were found in 100% of patients with acute symptoms and 60% of asymptomatic patients [19]. Not every study can achieve such remarkable results. In fact, it is very challenging to perform an awake laryngoscopy in a patient with acute respiratory distress. Furthermore, the topical application of local anesthetics to the airway before the actual laryngoscopic examination may further aggravate irritation of the vocal cords. Very often, during an acute dyspneic attack, the patient’s breathing pattern is chaotic with frequent unpredictable gasps and apneic pauses. The vocal cords may be readily seen in adduction, but it is not always straightforward to precisely correlate the adduction to the corresponding phase of respiration.
In asymptomatic patients, inhalation of methacholine has been used as a provocative test to precipitate an acute attack of VCD [36]. The diagnosis of VCD can be confirmed by flow-volume loops and flexible laryngoscopy performed during the provoked attack.
In cases in which patients know the exact irritant such as chlorine or ammonia which has been well documented to precipitate attacks, the same irritant may be used to provoke an acute attack instead of methacholine.
Differential Diagnoses
The clinical picture of VCD can be confused with many other clinical entities. A heightened awareness and a high index of suspicion are needed to make a correct diagnosis.
Asthma
The most important differential diagnosis in VCD is unquestionably an acute asthmatic attack. Many patients with VCD have been misdiagnosed with asthma for long periods of time and treated as such with bronchodilators and corticosteroids with poor results. The lack of therapeutic response has led erroneously to escalating doses of these drugs causing serious side effects. The distinguishing feature of VCD is the abnormal adduction of the cords during inspiration causing high pitched inspiratory stridor which is best heard over the larynx and upper trachea. In contrast, in asthma, the acute bronchoconstriction of the lower airways during exhalation causes expiratory wheezing which is heard loudest over the lower chest. VCD attacks usually occur suddenly and resolve rapidly, in contrast to asthmatic attacks which often become more severe over many hours or even days. Finally, in contrast to asthma, VCD does not respond to treatment with bronchodilators and steroids.
Laryngospasm
The clinical entity that most closely mimics VCD is laryngospasm. In contrast to the wide range of glottic narrowing encountered in VCD, the spasmodic adduction of the vocal cords in laryngospasm is much more intense and sustained through all phases of the respiratory cycle, leading to life-threatening complete airway obstruction. Physiologically, it is closely related to VCD. Laryngospasm has been considered as the most severe form of the glottic closure reflex. It may be precipitated by irritation of the vocal cords by secretions, acid reflux, food particles or blood. Laryngospasm is most commonly encountered in anesthesia practice, especially in pediatric patients during laryngeal manipulation under inadequate anesthesia or at emergence when the patient is still in the excitation phase with all airway reflexes in a hyperactive state. It may also result from hypocalcemia such as in hypoparathyroidism. Occasionally, laryngospasm may occur during sleep when severe gastric acid reflux irritates the vocal cords.
Vocal cord palsy
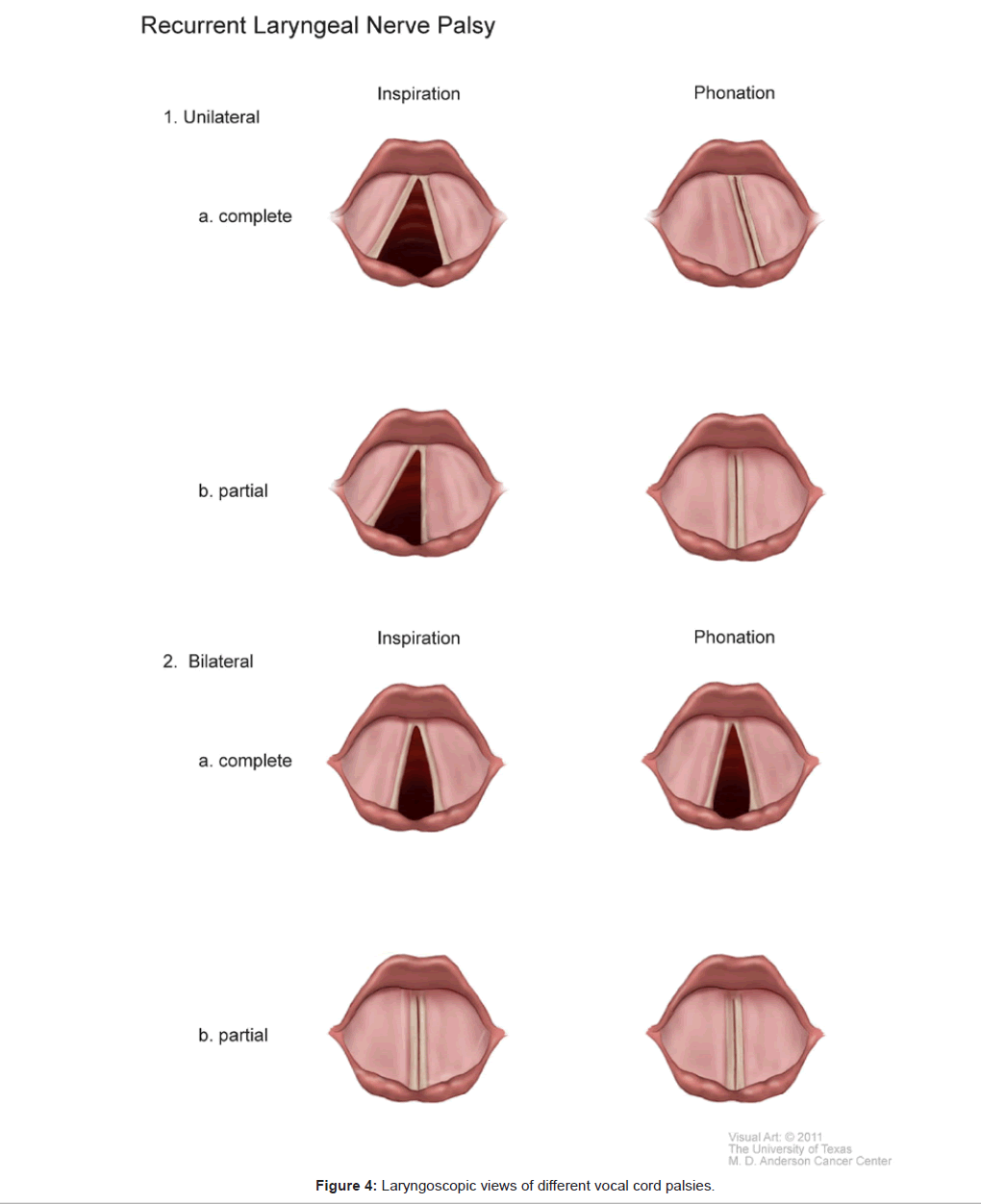
In contrast to VCD which is a purely functional abnormality of vocal cord motion, vocal cord palsy is secondary to an organic cause. Vocal cord palsy describes the decrease or absence of vocal cord motion following injury to the main vagus nerve or more commonly to its peripheral branches, the recurrent laryngeal nerve (RLN) and the superior laryngeal nerve (SLN). The most common causes of vocal cord palsy are surgical trauma, especially after procedures involving the thyroid, parathyroids, esophagus, and trachea. Other common causes include traumatic or prolonged tracheal intubations, malignant tumors and neurologic disorders such as amyotrophic lateral sclerosis and myasthenia gravis [37].
Injury to the SLN causes paralysis of the cricothyroid muscle, a vocal cord tensor. The voice becomes weak and husky, without dyspnea. Injuries to the RLN are more complex and potentially more serious with respect to respiration. The laryngeal muscles are not equally susceptible to injuries. Vocal cord abductors are more vulnerable than the adductors. Therefore, following a complete transection of the RLN causing paralysis of both abductors and adductors, the affected cord lies in the neutral position, midway between abduction and adduction. In this case, as the glottis cannot be closed, voice may be severely affected. Even though the glottic opening is slightly narrowed, there is still enough room to breathe. Under these circumstances, dyspnea occurs only in patients with pre-existing respiratory diseases such as severe chronic obstructive pulmonary disease. In contrast, a partial RLN injury results in a selective abductor paralysis, sparing the adductors. The affected vocal cord is adducted to the midline, severely narrowing the glottic opening. Thus, paradoxically, unilateral partial RLN palsy results in more severe dyspnea than complete RLN palsy, especially during exertion. This acute dyspneic attack can be confused with an attack of VCD. Bilateral RLN injuries are much more serious. In complete transection of bilateral RLNs, both cords are paralyzed, remaining motionless in the neutral position. Breathing through a narrowed glottis is still possible, albeit labored. On the other hand, in bilateral partial RLN injury, both cords are adducted to meet at the midline, completely closing the glottic opening with resulting respiratory obstruction.
During an acute dyspneic attack, in patients with vocal cord palsy, the cords do not move in contrast to the abnormal adduction of the cords seen with VCD. There is no paradoxical motion of the cords. The diagnosis of vocal cord palsy can be readily distinguished from VCD by history and by performing flexible laryngoscopy. Figure 4 illustrates the laryngoscopic views of different types of vocal cord palsies. In between attacks, the diagnosis of vocal cord palsy can be made by laryngeal electromyography, whereby the placement of electrodes into the intrinsic laryngeal muscles can assess their functional integrity [38].
Anaphylaxis
Following exposure to an allergen with massive release of histamine, anaphylactic shock results in the diffuse swelling of the tongue and larynx and severe bronchoconstriction. Symptoms of dyspnea, wheezing and chest tightness may lead to life-threatening complete airway obstruction. Anaphylaxis is characteristically accompanied by catastrophic cardiovascular collapse.
Angioedema
Angioedema involves the rapid swelling of dermal, subcutaneous and mucosal tissues. The skin of the face, oral mucosa, tongue, and throat can swell rapidly. In some cases progression to airway obstruction may occur. The acquired form of angioedema is caused by an allergic reaction and may be relieved by epinephrine, whereas in hereditary angioedema, epinephrine is not indicated.
Laryngomalacia
Laryngomalacia involves the softening of the epiglottis and arytenoid cartilages so that elongation of the epiglottis occurs with the walls folding in on themselves. The resultant floppiness of the cartilages leads to an omega-shaped epiglottis which characteristically prolapses over the glottic opening during inspiration, causing stridor.
Laryngeal pathology
Stridor may result from airflow obstruction at the level of the larynx from benign lesions such as vocal cord polyps or granulomas and from malignant lesions such as laryngeal squamous cell carcinoma or lymphoma.
Chronic medical diseases
Chronic systemic disorders with laryngeal involvement to consider in the differential diagnoses include rheumatoid arthritis, Wegener’s granulomatosis, progressive systemic sclerosis, lupus, and polychondritis. Patients with neuromuscular diseases such as amyotrophic lateral sclerosis may also present with symptoms similar to VCD. Infranuclear and supranuclear mechanisms are believed to play a role in the pathophysiology of glottic narrowing [39]. It is important to consider these diseases since they may be life-threatening and making the correct diagnosis and instituting appropriate treatment is essential.
Management
VCD is a complex clinical entity with multifactorial risks and precipitating factors. Consequently, a comprehensive multidisciplinary approach that encompasses many medical specialties is required for its effective management [40]. Medical specialists in respiratory medicine, allergy, otolaryngology, neurology, and psychiatry, as well as therapists in speech pathology, psychology, and occupational therapy can actively participate and offer important contributions to the management of these patients.
Treatment of Acute Episodes
Once organic causes have been ruled out and the diagnosis of VCD is made, symptomatic therapy is instituted to relieve the acute respiratory distress during attacks. A calm, reassuring, caring and supportive bedside manner is most conducive to the successful treatment of an acute episode of VCD [41,42]. Because VCD often has a component involving anxiety or panic, benzodiazepines such as midazolam titrated in incremental doses can be effective in relieving symptoms. Caution should be exercised to avoid oversedation and precipitation of respiratory failure. Creating distractions to shift the patient’s attention away from the dyspnea can often reduce the severity of the attack [43].
Various relaxed-throat breathing techniques have also been proposed as treatment for acute VCD. Panting, a breathing exercise in which patients are instructed to take rapid and shallow breaths, has shown good results with immediate relief of symptoms [44]. Panting stimulates contraction of the posterior cricoarytenoid muscle, a vocal cord abductor which counteracts vocal cord adduction and widens the glottic opening. Coaching the patient to make a long “s” sound during exhalation has also been tried with some success [45]. Other techniques described include breathing with pursed-lips and breathing through a straw. Continuous positive airway pressure has been used successfully to terminate acute attacks of VCD, presumably by slowing expiratory airflow and increasing expiratory time [46]. The resulting decrease in inspiratory time may help decrease the duration of paradoxical vocal cord adduction during inspiration.
The administration of heliox, a mixture of 80% helium and 20% oxygen, results in the rapid amelioration of acute symptomatic attacks, especially in patients with severe or persistent VCD [47]. As helium is much less dense than nitrogen, heliox is much less dense than air and decreases turbulent airflow through a narrowed opening. As airway resistance is reduced, work of breathing is significantly decreased. Furthermore as turbulence during inspiration is decreased, stridor is eliminated which also reduces the severe anxiety that often accompanies the acute attacks of VCD. By acting at the nerve terminals to prevent the release of acetylcholine, botulinum toxin type A induces muscle paralysis. It has been administered as an intralaryngeal injection [48] for severe cases of VCD to avoid the need for more invasive procedures such as intubation and tracheostomy. These latter interventions have been described only for rare refractory cases [49].
Long-Term Treatment and Prevention
Laryngeal control is essentially a behavior modification technique aimed to achieve voluntary control over motion of the vocal cords through specific breathing and speech techniques. To be effective, the patient’s complete understanding, trust and active participation is absolutely required. Unfortunately, many VCD patients may have difficulty understanding and accepting speech therapy alone without adjunct pharmacological agents as the treatment option for their highly distressing symptoms. Furthermore, large randomized clinical trials are sorely lacking due to the relative rarity of VCD. Despite these reservations and limitations, it is widely accepted that speech therapy constitutes the cornerstone of treatment of this disorder.
Long-term management and prevention of VCD requires a multidisciplinary approach to control the multitude of underlying psychological and organic disorders involved in its pathogenesis. Thus, psychiatric illnesses such as severe anxiety, major depression, and posttraumatic stress syndrome must be addressed with patient education, reassurance, supportive counseling, psychotherapy and psychotropic medications. The use of inhaled anticholinergics such as ipratropium has been reported to successfully prevent exercise-induced VCD [50]. Similarly, conditions such as chronic post-nasal drip, severe gastroesophageal reflux, and chronic exposure to airborne irritants should be identified, eliminated and optimally treated. These noxious agents likely cause irritation, inflammation, and edema leading to hyperreactivity of the vocal cords. Avoidance of these irritants would logically improve VCD, yet there are no large-scale prospective studies that prove the efficacy of irritant avoidance as a successful cure or prevention for VCD. Environmental irritants may also be difficult to avoid completely. Furthermore, the etiologies of VCD are usually multifactorial and the elimination of one single factor may not eradicate symptoms.
Long-term speech therapy, behavioral therapy with progressive muscle relaxation techniques, and controlled breathing exercises have been found to be extremely valuable in helping patients understand and control the paradoxical movement of the vocal cords. In one study, 95% of patients treated with speech therapy were able to control their symptoms [51]. Once patients have achieved proficiency in these techniques, gradual controlled challenges with known triggering irritants to elicit VCD symptoms allow patients to control their breathing patterns and to voluntary terminate acute dyspnea attacks.
Conclusion
The abnormal motion of the vocal cords has been described by many different medical specialties including allergy, respiratory medicine, and otolaryngology. Attacks of dyspnea accompanied by the noisy breathing of VCD can be mimicked by many other clinical entities. This confusion has been compounded by the lack of standardization in reporting results of investigational procedures such as spirometry and laryngoscopy. An increased awareness and understanding of VCD is essential to prevent the misdiagnosis and consequent mismanagement of VCD as intractable asthma. Further research focusing on pathogenesis, standard criteria for diagnosis, and comprehensive treatment protocols are warranted to achieve optimal care of patients with VCD.
References
- Dunglison RD (1842) The Practice of Medicine. Lea and Blanchard, Philadelphia, PA 257-258.
- Patterson R, Schatz M, Horton M (1974) Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy 4: 307-310.
- Lund DS, Garmel GM, Kaplan GS, Tom PA (1993) Hysterical stridor: a diagnosis of exclusion. Am J Emerg Med 11: 400-402.
- Smith ME, Darby KP, Kirchner K, Blager FB (1993) Simultaneous functional laryngeal stridor and functional aphonia in an adolescent. Am J Otolaryngol 14: 366-369.
- Dailey RH (1976) Pseudoasthma: a new clinical entity? JACEP 5: 192-193.
- Downing ET, Braman SS, Fox MJ, Corrao WM (1982) Factitious asthma. Physiological approach to diagnosis. JAMA 248: 2878-2881.
- Rodenstein DO, Francis C, Stanescu DC (1983) Emotional laryngeal wheezing: a new syndrome. Am Rev Respir Dis 127: 354-356.
- Barnes SD, Grob CS, Lachman BS, March BR, Loughlin GM (1986) Psychogenic upper airway obstruction presenting as refractory wheezing. J Pediatr 109: 1067-1070.
- Ramirez JR, Leon I, Rivera LM (1986) Episodic laryngeal dyskinesia. Clinical and psychiatric characterization. Chest 90: 716-721.
- Gallivan GJ, Hoffman L, Gallivan KH (1996) Episodic paroxysmal laryngospasm: voice and pulmonary function assessment and management. J Voice 10: 93-105.
- Morrison M, Rammage L, Emami AJ (1999) The irritable larynx syndrome. J Voice 13: 447-455.
- Christopher KL, Wood RP II, Eckert RC, Blager FB, Raney RA, et al. (1983) Vocal-cord dysfunction presenting as asthma. N Engl J Med 308: 1566-1570.
- Patel NJ, Jorgenson C, Kuhn J, Merati AL (2004) Concurrent laryngeal abnormalities in patients with paradoxical vocal fold dysfunction. Otolaryngol Head Neck Surg 130: 686-689.
- Newman KB, Dubester SN (1994) Vocal cord dysfunction: masquerader of asthma. Semin Respir Crit Care Med 15: 161-167.
- Hicks M, Brugman SM, Katial R (2008) Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care 35: 81-103.
- Jain S, Brandi V, Zimmerman J (1997) Incidence of vocal cord dysfunction in patients presenting to emergency room with acute asthma exacerbation. Chest 11:243.
- Morris MJ, Grbach VX, Deal LE, Boyd SY, Morgan JA, et al. (2002) Evaluation of exertional dyspnea in the active duty patient: the diagnostic approach and the utility of clinical testing. Mil Med 167: 281-288.
- Rundell KW, Spiering BA (2003) Inspiratory stride in elite athletes. Chest 123: 468-474.
- Newman KB, Mason UG, Schmaling KB (1995) Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med 152: 1382-1386.
- Brugman S (2003) The many faces of vocal cord dysfunction. What 36 years of literature tells us. Am J Respir Crit Care Med 167: A588.
- Omland T, Brondbo K (2008) Paradoxical vocal cord movement in newborn and congenital idiopathic vocal cord paralysis: two of a kind? Eur Arch Otorhinolaryngol 265: 803-807.
- Sullivan MD, Heywood BM, Beukelman DR (2001) A treatment for vocal cord dysfunction in female athletes: an outcome study. Laryngoscope 111: 1751-1755.
- Rundell KW, Spiering BA (2003) Inspiratory stridor in elite athletes. Chest 123: 468-474.
- Murkami Y, Kirchner JA (1972) Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhino Laryngol 81: 59-72.
- Bucca C, Rolla G, Brussino L, De Rose V, Bugiani M (1995) Are asthma-like symptoms due to bronchial or extrathoracic airway dysfunction? Lancet 346:791-795.
- Ayres JG, Gabbott PL (2002) Vocal cord dysfunction and laryngeal hyperresponsiveness: a function of altered autonomic balance? Thorax 57: 284-285.
- Bucca C, Rolla G, Scappaticci E, Chiampo F, Bugiani M, et al. (1995) Extrathoracic and intrathoracic airway responsiveness in sinusitis. J Allergy Clin Immunol 95: 52-59.
- Harding SM (2003) Recent clinical investigations examining the association of asthma and gastroesophageal reflux. Am J Med 115 Suppl 3A: 39S-44S.
- Perkner JJ, Fennelly KP, Balkissoon R, Bartelson BB, Ruttenberg AJ, et al. ( 1998) Irritant-associated vocal cord dysfunction. J Occup Environ Med 2: 136-143.
- Galdi E, Perfetti L, Pagella F, Bertino G, Ferrari M, et al. (2005) Irritant vocal cord dysfunction at first misdiagnosed as reactive airway dysfunction syndrome. Scand J Work Environ Health 31: 224-226.
- Bhargava S, Panitch HB, Allen JL (2000) Chlorine induced paradoxical vocal cord dysfunction. Chest 118: 295S-296S.
- Walaschek C, Forster J, Echternach M (2010) Vocal cord dysfunction without end? Klin Padiatr 222: 84-85.
- Goldstein R, Bright J, Jones SM, Niven RM (2007) Severe vocal cord dysfunction resistant to all current therapeutic interventions. Respir Med 101: 857-858.
- Hayes JP, Nolan MT, Brennam N, Fitzgerald MX (1993) Three cases of paradoxical vocal cord adduction followed up over a 10-year period. Chest 104: 678-680.
- Eggleston PA, Ward BH, Pierson WE, Bierman CW (1974) Radiographic abnormalities in acute asthma in children. Pediatrics 54: 442- 449.
- Guss J, Mirza N (2006) Methacholine challenge testing in the diagnosis of paradoxical vocal fold motion. Laryngoscope 116: 1558-1561.
- Benninger MS, Gillen JB, Altman JS (1998) Changing etiology of vocal fold immobility. Laryngoscope 108: 1346-1350.
- Miller RH, Rosenfield DB (1983) The role of electromyography in clinical laryngology. Otolaryngol Head and Neck Surg 92: 287-291.
- Van der Graaff MM, Grolman W, Westermann EJ, Boogaardt HC, Koelman H, et al. (2009) Vocal cord dysfunction in amyotrophic lateral sclerosis: four cases and a review of the literature. Arch Neurol 66: 1329-1333.
- Christopher KL, Morris MJ (2010) Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin North Am 43: 43-66.
- George MK, O’Connell JE, Batch AJ (1991) Paradoxical vocal cord motion: an unusual cause of stridor. J Laryngol Otol 105: 312- 314.
- Kisson N, Kormick JB, Frewen TC (1988) Psychogenic upper airway obstruction. Pediatrics 81:714- 717.
- Wood RP, Milgrom H (1996) Vocal cord dysfunction. J Allergy Clin Immunol 98: 481-485.
- Pitchenik AE (1991) Functional laryngeal obstruction relieved by panting. Chest 100: 1465-1467.
- Brugman SM, Simon SM (1998) Vocal cord dysfunction: don’t mistake it for asthma. Phys Sportsmed 26: 63-85.
- Goldman J, Muers M (1991) Vocal cord dysfunction and wheezing. Thorax 46: 401-404.
- Weir M (2002) Vocal cord dysfunction mimics asthma and may respond to heliox. Clin Pediatr 41: 37.
- Maillard I, Schweizer V, Broccard A, Duscher A, Liadet L, et al. (2000) Use of botulinum toxin type A to avoid tracheal intubation or tracheostomy in severe paradoxical vocal cord movement. Chest 118: 874-877.
- Park DP, Ayres JG, McLeod DT, Mansur AH (2007) Vocal cord dysfunction treated with long-term tracheostomy: 2 case studies. Ann Allergy Asthma Immunol 98: 591-584.
- Doshi DR, Weinberger MM (2006) Long-term outcome of vocal cord dysfunction. Ann Allergy Asthma Immunol 96: 794-799.
- Sullivan MD, Heywood BM, Beukelman DR (2001) A treatment for vocal cord dysfunction in female athletes: an outcome study. Laryngoscope 111: 1751-1755.
Citation: Truong A, Truong DT (2011) Vocal Cord Dysfunction: An Updated Review. Otolaryngol S1:002. DOI: 10.4172/2161-119X.S1-002
Copyright: © 2011 Truong A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 29428
- [From(publication date): 3-2011 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 23830
- PDF downloads: 5598