Research Article Open Access
Vectoral Control Synergy: Combined Use of ITN and Residual House Spray in the Control of Malaria in South Western Nigeria
Afolabi OT1*, Onayade AA1, Sule SS2 and Olajide FO1
1Department of Community Health, Obafemi Awolowo University, Ile-Ife, Nigeria
2National Postgraduate Medical College, Ijaniki, Lagos, Nigeria
- *Corresponding Author:
- Dr. Olusegun Tope Afolabi,
Department of Community Health
OAU, Ile-Ife, Nigeria
Tel: 234-803-388-5447
E-mail: temitopesegun@yahoo.com
Received date: June 18, 2012; Accepted date: July 17, 2012; Published date: July 19, 2012
Citation: Afolabi OT, Onayade AA, Sule SS, Olajide FO (2012) Vectoral Control Synergy: Combined Use of ITN and Residual House Spray in the Control of Malaria in South Western Nigeria. J Community Med Health Educ 2:161. doi:10.4172/2161-0711.1000161
Copyright: © 2012 Afolabi OT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Introduction: To control malaria, better techniques to apply existing control methods and improved tools must be developed. Both ITNs and residual spraying have been found to be equally effective when used singly, however, it would be worthwhile to determine any synergistic effect or otherwise of combined ITNs and residual spraying in prevention of malaria. The aim of this study is to compare the combined effects of ITNs and residual spraying on the control of malaria as opposed to ITN alone among school children.
Methodology: This was an open randomised community trial conducted at the Federal Government Girls College (a school with enrollees from all parts of the Country), Ipetumodu, Osun State in South-Western Nigeria within a three month period. All students who had spent at least six months were eligible to participate.
Result: Seven hundred and sixty three participants were recruited; baseline prevalence of malaria was 81%. Cumulative incidence of malaria was 185/1000 and 215/1000 in the dual and single intervention groups respectively yielding a relative risk of 0.88 (95%CI 0.65-1.14). The prevalence of parsitaemia reduced significantly by 53% and 45% in the dual and single intervention groups respectively compared to baseline. There was a significant reduction in vector density between the two groups (p=0.04) with a 51% and 17% drop in the dual and single intervention groups respectively.
Conclusion: The dual intervention group had a huge impact on vector density but did not significantly affect the level of parsitaemia and incidence of malaria.
Keywords
Malaria; ITN; Residual house spray; Control
Introduction
Malaria is by far the world’s most important parasitic disease and is responsible for 8.2% of disability-adjusted life years (DALYs) [1]. It exacts an enormous toll on lives in medical costs and in days of labour lost [2]. The estimated costs of malaria in terms of strain on health systems and economic activity lost are enormous amounting to about US $10 billion to $12 billion every year in lost Gross Domestic Product (GDP) [3].
It is still a public health problem today in more than 90 countries with an estimated worldwide incidence of about 300-500 million cases each year and a mortality of over 1million deaths per annum, significant proportion of which occurs in children under five years of age and pregnant women [2].
Malaria control has gone round a full cycle from control to eradication and back to control. Attempts at eradication via the use of insecticide spray began shortly after the discovery of dichloro diphenyl trichloroethane (DDT) [4]. This chemical vector control which was combined with chemotherapy and surveillance have not largely met with the desired objectives due to toxicity from persistence in the environment, resistance of vectors and resistance of parasites to drugs [4,5]. This approach has since run out of favour and its use is now restricted to specific high risk and epidemic prone areas [6]. However, newer methods based on the usefulness of pyrethoids from naturally occurring plants (Crysanthemum spp) and its synthetic products as residual insecticides has brought renewed hope.
Vector control at community or village level has a better chance of success than vertically organised control programmes and the World Health Organisation (WHO) now recommends its incorporation into primary health care (PHC) [5]. Some United Nations agencies through multilateral initiatives initiated a new strategy, “Roll Back Malaria” (RBM) whose goal is to halve the malaria burden in participating countries by year 2010 applying a multipronged protection involving use of insecticide treated materials (ITNs), environmental management to control mosquitoes and strengthening research capabilities among others [7].
Multicentre randomised controlled field trials in Africans suggest that overall childhood mortality can be lowered by 15-35% through the use of ITNs [8] and some non-randomised controlled studies in school children found a 60% reduction in febrile episodes with use of ITNs [9-12].
Some studies suggest they are also associated with mass killing effect with measurable decreases in the density of mosquito vector population while other studies show little evidence for such an effect [13,14].
House spraying with residual insecticides have been found to be equally as effective as ITN in malaria control [15]. Since residual house spraying (RHS) is a good environmental control method and with introduction of easily biodegradable, non-persistent, ecofriendly insecticides like Permethrin to replace DDT. Thus, to control malaria, better ways must be found to apply existing control methods, improved tools must be developed and solutions must be identified to circumvent and combat emerging problems.
Both ITNs and residual spraying have been found to be equally effective when used singly, however, it would be worthwhile to determine any synergistic effect or otherwise of combined ITNs and residual spraying in prevention of malaria.
The aim of this study is to compare the combined effects of ITNs and residual spraying on the control of malaria as opposed to ITN alone among school children.
Materials and Methods
The study was conducted at the Federal Government Girls College (a school with enrollees from all parts of the Country), Ipetumodu, in Ife North Local Government area of Osun State in South-Western Nigeria within a three month period. This location is holoendemic for malaria as are most part of Nigeria with stable malaria transmission all year round and a peak incidence during the rainy season, which stretches from April to October. The school is located on the outskirt of town and has a student population of about 1200 pupils who are all boarders. There are five dormitories each housing an average of 250 pupils. The dormitories are situated within 100m of one another with net protection on the windows; these nets are however mostly worn out.
This was an open randomised community trial. The students were randomised by hostels as the primary sampling unit. The five (5) dormitories in the school were randomly assigned to groups by simple balloting. The first two dormitories picked by ballot were assigned to study Group A which had dual intervention of insecticide treated bed nets and the houses sprayed with residual insecticide, while the next two dormitories were assigned to Group B and had ITNs alone without spraying. The last dormitory was reserved to accommodate any pupil who reacted to the intervention or declined to participate in the study.
Sample size was determined using the formula for estimating independent proportions [16]. With level of significance set at 95%, power at 80% and prevalence of malaria at 15% [17] a sample size of 355 per group was obtained.
All registered students of the school and who had been students of the school for at least six months and who were willing to participate in the study were included in the study while any pupil who refused to participate in the study or who was on prophylactic antimalarial drugs; and any pupil who reacted adversely to any of the intervention was excluded from the study.
Written consent was sought and obtained from the school authority, parents and assent from the pupils. The house-mistresses and sick-bay nurses were briefed and enlisted in the study to ensure compliance and reduce attrition. Ethical clearance was obtained from the Ethics and Research Committee of the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife.
All students were informed about the study and enlightened on the usefulness of using their bed nets regularly. They were also educated on why they should not wash the nets during the lifespan of the study. They were told about the spraying of the rooms and were asked to report if they noticed any untoward effects after the spraying.
The study population who were already assigned to two groups by random sampling were taught how to hang the bed nets and then allocated bed nets which were treated by dipping into aqueous emulsion of α-cypermethrin to yield an application rate of 0.5g/m2 and subsequently dried in a shade. Compliance with use of bed nets was ensured by the house mistress and prefects.
The two study dormitories in group A were then sprayed with α-cypermethrin (50% emulsion) spray dilutions adjusted to give target dosage of 2g/m2 on the wall surface.
Baseline data was collected by taking blood smears from all the student population to ascertain the prevalence of malaria in the school. All students that had a positive blood film were treated with a single dose of sulfadoxine-pyrimethamine.
Vectoral density was assessed by applying a knock-down pyrethrum spray catch to the dormitories. The number of mosquitoes and the proportion that had blood meal was assessed.
Outcome measures were; prevalence of parasitaemia which was assessed by taking a blood film from a subset of the student population. A blood film was taken by finger prick using a lancet and made into a thick blood film, which was obtained by staining with 1/30 dilution of stock Giemsa for 45minutes and read as described by Leenstra et al. [18]. For validity of the measure, 100 replicate measurements were read by the researcher and another 50 randomly selected samples were read and compared with the readings of a consultant parasitologist.
Blood film reading was validated with Pearson’s correlation of 0.84 and 0.90 for inter and intra-observer variation and Kappa statistic for degree of agreement of 0.79 and 0.82 for inter and intra-observer agreement.
Incidence of malaria: A prospective follow up of the students in the two groups was carried out over three months to ascertain the number of students in each group that developed malaria using clinical and hematological assessments and results are entered into a proforma. Every student who presented at the sick bay with symptoms suggestive of malaria was examined.
A case of malaria in this study was defined as any pupil living in the study area presenting with typical febrile paroxysm or any other symptom consistent with malaria, such as, isolated intermittent fever >37.5°C (axillary), headache or other body pain; digestive symptoms and sweating beginning in the period not more than 2 weeks before the diagnosis. Post intervention prevalence of malaria was assessed using simple random sampling technique to select 100 students each from both study groups. Vectoral density was defined as the number of mosquitoes collected in the dormitories using a knock-down insecticide spray catch as a proxy because an exit trap could not be used due to poor state of the window nets. This was assessed at the first and second month post-intervention.
Data collected from the field was analysed using the Statistical Programme for Social Sciences version 12 (SPSS, Chicago, Illinois) and Computer Programme for Epidemiologists (WINPEPI). Prevalence of parasitaemia was presented using tables and Chi-square values calculated. Incidence of malaria was reported by simple frequencies and appropriate charts and relative risks calculated.
Vectoral density was assessed by absolute total catch of mosquitoes and proportion of mosquitoes that had blood meal. Test of association was done using Chi-square and Fisher’s exact test was used when cells had expected counts less than five. P was significant at <0.05.
Results
A total of 763 participants were recruited into the study; 373 (48.8%) were in study group A which had dual intervention of ITN and RHS while 390 (51.2%) were in study group B with ITN alone. Table 1 shows the socio-demographic characteristics of the participants by study groups. The distribution of the participants by age was not significantly different between the two groups (p=0.37). Majority of the participants were Christians in both study groups with 54.7% in group A and 53.8% in group B.
| Group A (ITN + RHS) N=373 (%) |
Group B (ITN only) N=390 (%) |
χ2 | P value | ||
|---|---|---|---|---|---|
| Age | |||||
| 9-11 | 118 (31.6) | 134 (34.4) | 2.01 | 0.37 | |
| 12-14 | 170 (45.6) | 158 (40.5) | |||
| 15-17 | 85 (22.8) | 98 (25.1) | |||
| Religion | |||||
| Christianity | 204 (54.7) | 210 (53.8) | 3.08 | 0.22 | |
| Islam | 145 (38.9) | 165 (42.3) | |||
| Others | 24 (6.4) | 15 (3.8) | |||
| Ethnicity | |||||
| Yoruba | 252 (67.6) | 262 (67.2) | 2.32 | 0.51 | |
| Igbo | 93 (24.9) | 102 (26.2) | |||
| Hausa | 6 (1.6) | 2 (0.5) | |||
| Others | 22 (5.8) | 24(6.2) | |||
| Educational status | |||||
| Senior secondary Junior secondary | 140 233 | 148 242 | 0.014 | 0.90 | |
Table 1: Socio-demographic characteristic of the study population.
Although this is a Federal school, Yorubas were the predominant ethnic group in this population with about 67% in both study groups while the Igbos were about 25%. This distribution is not totally unexpected considering the region of the country the school is located. What is however striking is the almost equally proportionate distribution in the two study groups (p=0.51) which presupposes that the school may have preset criteria for allocating students into different hostels.
Parasitaemia was assessed at baseline. Of the 373 students in study group A, 69 (18.2%) had no malaria parasite in the peripheral blood while 261 (70%) had mild (+) parasitaemia, 39 (10.5%) had moderate (++) parasitaemia and 4 (1.1%) had severe (+++) parasitaemia. While of the 390 students in study group B, 71 (18.2%) had no parasite in the peripheral blood, 253 (64.9%) had mild (+) parasitaemia, 64 (16.4%) had moderate (++) parasitaemia and 2 (0.5%) had severe (+++) parasitaemia. Thus the prevalence of parasitaemia in both study groups at baseline was about 81% as presented in Table 2.
| Group A (ITN+RHS) N=373 (%) | Group B (ITN alone) N=390 (%) | |
|---|---|---|
| Nil | 69 (18.5) | 71 (18.2) |
| + | 261 (70.0) | 253 (64.9) |
| ++ | 39 (10.5) | 64 (16.4) |
| +++ | 4 (1.0) | 2 (0.5) |
Table 2: Baseline prevalence of parasitaemia in the study groups.
The baseline entomological index for mosquito density is shown in Table 3 where the number of mosquitoes collected with pyrethrum spray catch pre-intervention was 42 in study group A and 38 in study group B of the 42 mosquitoes in group A, 8 (19%) had taken blood meals compared to 13 (34%) in study group B which was not statistically significant (p=0.12).
| Group A (ITN+RHS) N (%) | Group B (ITN alone) N (%) | |
|---|---|---|
| Fed | 8 (19) | 13 (34) |
| Not Fed | 34 (81) | 25 (66) |
| Total | 42 (100) | 38 (100) |
χ2=2.369; p=0.124
Table 3: Baseline entomological indices in the study groups.
Table 4 shows the cumulative incidence of clinical malaria in both study groups. In study group A, there were 69 cases of clinical malaria with incidence of 185/1000 population while in group B there were 84 cases of clinical malaria with incidence of 215/1000 population. The relative risk of malaria comparing study group A to B was 0.86 (95% CI 0.65-1.14). The protective efficacy of the dual intervention (ITN+RHS) is 14% [100(1-RR)].
| Study Group | Clinical Malaria | Total | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| A (ITN + Spray) | 69 (18.5) | 304 (81.5) | 373 (100) |
| B (ITN alone) | 84 (21.4) | 306 (78.6) | 390 (100) |
| Total | 153 (20.1) | 610 (79.9) | 763 (100) |
RR=0.86 (0.65-1.14)
Table 4: Cumulative Incidence of Clinical Malaria in the Two Study Groups.
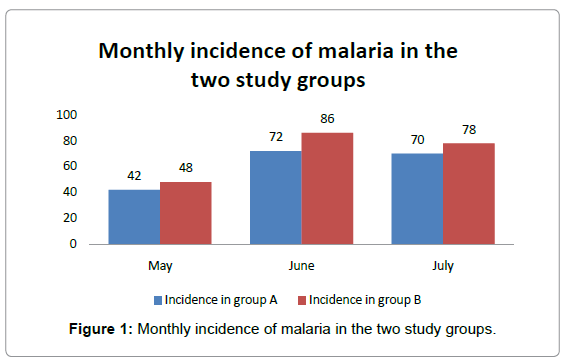
Figure 1 shows that there is consistently greater number of cases of malaria in study group B than group A.
The prevalence of parasitaemia had dropped to 28% in group A and 36% in group B (Table 5). Although the difference was not significant between the two groups (χ2=1.47; p=0.23), the interventions had reduced prevalence of parasitaemia by about 53% in group A and 45% in group B compared to baseline prevalence. These drops were significant in both groups [χ2 = 107.88 p=<0.001 in group A and χ2 = 83.62 p=<0.001 in group B] as shown in Tables 6 and 7 respectively.
| Level of Parasitaemia | Group A (ITN+RHS) N=100 (%) | Group B (ITN alone) N=100 (%) |
|---|---|---|
| Nil | 72 (72.0) | 64 (64.0) |
| + | 21 (21.0) | 24 (24.0) |
| ++ | 7 (7.0) | 11 (11.0) |
| +++ | 0 (0.0) | 1 (1.0) |
Table 5: Post intervention parasitaemia prevalence in the study groups.
| Group A (ITN +RHS) | Malaria parasitaemia Yes (%) | Malaria parasitaemia No (%) | Total |
|---|---|---|---|
| Pre Intervention | 304 (81.5) | 69 (18.5) | 373 (100) |
| Post Intervention | 28 (28) | 72 (72) | 100 (100) |
| Total | 322 (68.1) | 171 (31.9) | 473 (100) |
χ2 = 107.88 p=<0.001
Table 6: Pre and post intervention parasitaemia in study group A.
| Group B (ITN alone) | Malaria parasitaemia Yes (%) | Malaria parasitaemia No(%) | Total (%) |
|---|---|---|---|
| Pre Intervention | 319 (81.8) | 71 (18.2) | 390 (100) |
| Post Intervention | 36 (36) | 64 (64) | 100 (100) |
| Total | 355 (72.5) | 135 (27.5) | 490 (100) |
χ2 = 83.62 p=<0.001
Table 7: Pre and post intervention parasitaemia in study group B.
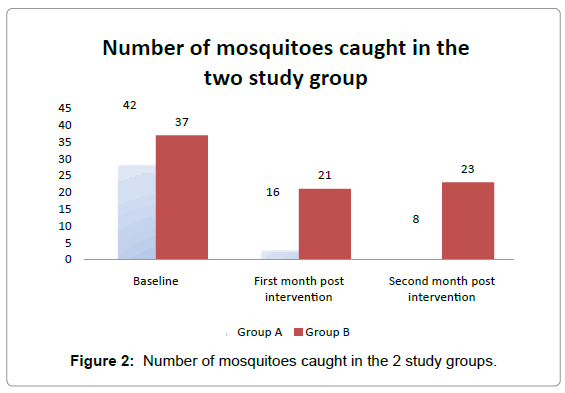
Figure 2 shows the number of mosquitoes collected with pyrethrum spray catch. At one month post intervention, 16 mosquitoes were collected in study group A while 21 mosquitoes were collected in study group B. At two months post intervention, the mosquito density in group A had reduced to 8 while group B was 23. This represents a 51% and 17% reduction in vectoral density in groups A & B respectively. The decrease in mosquito density was significantly different between study group A and B (χ2= 6.480; p=0.04). In study group A, 2 (8.3%) mosquitoes compared to 6 (13.8%) in study group B had blood meal. This difference was not statistically significant (χ2=0.065; p=0.79). When these values were compared with the baseline values there were significant differences (Extended MH χ2 =31.89; p <0.05) table not shown.
Discussion
The RBM initiative has been emphasizing the need to look at improved tools and modification of existing tools to better achieve malaria control [3]. This need prompted this study which assessed possible synergistic effect of ITN and RHS on the control of malaria. In reviewing relevant literature, no study similar to this has been conducted in terms of study population and the main objectives. The only study in the literature on adolescents was that conducted by Leenstra and co-workers on ITNs among secondary school students in Kenya [18].
The prevalence of parasitaemia in this study at baseline was 81% which is very high when compared to the 28% baseline parasitaemia reported by Leenstra et al. in a randomized controlled trial of Insecticide treated bed nets in Western Kenya [18]. The disparity may be due to the different endemicity levels in the two study areas which is further strengthened by findings from a study in Kenya by Zhon G et al. that reported a baseline prevalence of 73.5% in the valley and 43.8% in the highland regions [19]. Our finding is close to 64% reported by Wilson and co workers in a survey conducted in South Africa amongst 11-15 year olds [20]. According to Smith et al. [21] this high prevalence could be attributed to the acquired immunity which he postulated had little effect on infection rates but protects against developing full blown malaria such that individual inoculations transiently add to point prevalence irrespective of age. Thus, slow accumulation of small effects explains why the maximum effect on prevalence is in older children [21].
The incidence of malaria in study group A was less than that in study group B with a protective efficacy of 14%. The difference was however not significant, suggesting a modest effect of the combined use of ITN and RHS as opposed to ITN alone. This is similar to 12% reported by Protopopoff and co-workers in Burundi which was also not significant [22]. This effect could be due to the mass killing effect of the insecticide as suggested by Howard et al. [23] in a community randomized trial in the highlands of Kenya and Guyatt et al. [24] in another community randomized trial in Kenya. The difference may also be accounted for by a reduction in inoculation rate when not under bed nets as the residual spray due to the excito-repellant effect may reduce the density of the mosquito within the room feeding around dusk as shown by Reisen and colleagues [25].
Snow et al. [26] in a study carried out in Gambia showed that permethrin treated bed nets are more effective in preventing heavy infections and those resulting in clinical episodes than preventing infections overall. A possible explanation according to Snow and coworkers is that the exposure to permethrin reduces in some way the average sporozoite load from infected mosquitoes.
The prevalence of parasitaemia at the end of the study reduced by 53% in group A and 45% in group B. This is much lower compared with the study by Guyatt et al. [24] which reported a reduction in infection by 75% for RHS alone and 63% for ITN alone.
Mosquito density in this present study was reduced significantly in both study groups when compared to baseline figures. Similar results were obtained in a study by Quinones et al. [27] in Gambia where the mosquito density was reduced by about 75% and Protopopoff et al. [28] who reported a 56% reduction in vector density in Burundi. The authors attributed this to a probable mass killing effect or the excitorepellant characteristic of permethrin.
The effect of the interventions on feeding patterns of mosquito is evident in this study where the proportion of mosquitoes that had blood meals was significantly reduced, though not between the two study groups. Genton et al. [29] had shown in his study that despite adequate coverage of bed nets, mosquitoes had still managed to find a human host. The presence of the permethrin may make the mosquitoes rest outdoors and the feeding may have been done outside. A testimony to this assertion is that in study group A there were fewer mosquitoes that had had blood meals compared to study group B.
Conclusion
This study has assessed the effect of combined use of ITN and RHS as opposed to ITN alone in the control of malaria among secondary school students in Ife North Local Government Area Osun State. The result of this study demonstrated that a combination of ITN and RHS was able to control malaria as the incidence of malaria dropped, though not significantly different from the use of ITNs alone. The prevalence of parasitaemia also went down significantly with the dual intervention but did not show any statistically significant advantage over ITN alone.
The most striking effect was on the entomological indices where the dual intervention was significantly better than ITN alone in reducing mosquito density and proportion of mosquitoes that had taken blood meal.
Recommendations
In view of the findings in this study, the following recommendations are hereby made.
i. Combination of ITN and RHS should be used in the study centre and communities that are holoendemic for malaria where vectoral density is high especially in communities where mosquitoes tend to rest indoor after feeding.
ii. Policy encouraging use of malaria control measures such as ITN or ITN/RHS in schools should be formulated as this will reduce the incidence of malaria.
Acknowledgements
The authors will like to appreciate the effort of the management team of the Federal Government Girls College Ipetumodu for their cooperation in conducting this study particularly the Head Nurse in the Sick bay and the House Mistresses.
References
- WHO (2004) Global burden of disease:2004 update accessed 11/7/12
- Roll Back Malaria: Country updates; October 1998-June 2000 WHO/CDS/RBM/2000: 24 page 35.
- Key malaria facts Accessed from the "Roll Back Malaria" website on 16/5/12.
- Gilles HM (1993) History of malaria In Bruce Chwatt’s Essential malariology. 3rd Edition, William Heinemann, London: 1-4.
- WHO (1983) Integrated vector control Seventh report of the WHO Expert Committee on vector biology and control. Geneva.
- WHO (1998) TDR Progress 1997-98: 14th programme Report. Geneva.
- WHO (2001) Roll Back Malaria: Country strategies and resource equipment. WHO/CDS/RBM/ 34 page 4.
- Nevill CG, Some ES, Mungala VO, Mutemi W, New L, et al. (1996) Insecticide treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coasts. Trop Med Int Health 1: 139-146.
- Abdulla S, Schellenberg JA, Nathan R, Mukasa O, Marchant T, et al. (2001) Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2years in Tanzania: community cross sectional study. BMJ 322: 270-273.
- Premji Z, Lubega P, Hamisi Y, Mchopa E, Minjas J, et al. (1995) Changes in malaria associated morbidity in children using insecticide treated mosquito nets in the Bagamoyo district of coastal Tanzania. Trop Med Parasitol 46: 147-153.
- Beach RF, Ruebush TK 2nd, Sexton JD, Bright PL, Hightower AW, et al. (1993) Effectiveness of permethrin impregnated bed nets and curtains for malaria control in a holoendemic area of western Kenya. Am J Trop Med Hyg 49: 290-300.
- Lindsay SW, Gibson ME (1988) Bed nets revisited - old idea, new angle. Parasitology Today 4: 270-272.
- Megesa SM, Wikes TJ, Mnzava AE, Njunwa KJ, Myamba J, et al. (1991) Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. part 2. Effects on the malaria vector population. Acta Trop 49: 97-108.
- Charlwood JD, Graves PM (1987) The effect of permethrin impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Med Vet Entomol 1: 319-327.
- Goodman CA, Mnzava AE, Dlamini S, Sharp BL, Mthembu DJ (2001) Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in Kwazulu-Natal, South Africa. Trop Med Int Health 6: 280-295.
- Zar JH (1998) Biostatistical analysis, (4thedn), Prentice Hall.
- Steketee RW, Nahlen BL, Parise ME, Menendez C (2001) The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64: 28-35.
- Leenstra T, Phillip-Howard PA, Kanliki SK, Hawley WA, Alaii JA, et al. (2003) Permethrin-treated bed nets in the prevention of malaria and anaemia in adolescent school girls in Western Kenya. Am J Trop Med Hyg 68: 86-93.
- Zhon G, Githeko AK, Minakawa N, Yan G (2010) Community-wide benefits of targeted indoor residual spray for malaria control in the western Kenyan highlands. Malar J 9: 67.
- Wilson DB, Garnham PC, Swellengrebel NH (1950) A review of hyperendemic malaria. Trop Dis Bull 47: 677-698.
- Smith T, Hii J, Genton B, Muller I, Booth M, et al. (2001) Associations of peak shifts in age prevalence for human malaria with bed net coverage. Trans R Soc Trop Med Hyg 95: 1-6.
- Protopopoff N, Van Herp M, Maes P, Reid T, Baza D, et al. (2007) Vector control in a malaria epidemic occurring with a complex emergency situation in Burundi: a case study. Malar J 6: 93.
- Howard SC, Omumbo J, Nevill C, Some ES, Donnelly CA (2000) Evidence for a mass community effect of insecticide-treated bednets on the incidence of malaria on the Kenyan coast. Trans R Soc Trop Med Hyg 94: 357-360.
- Guyatt HL, Corlett SK, Robinson TP, Ochola SA, Snow RW (2002) Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticide-treated bed nets. Trop Med Int Health 7: 298-303.
- Reisen WK, Aslamkhan M (1978) Biting rhythm of some Pakistan mosquitoes (Diptera: Culicidae). Bull Entomol Res 68: 313-330.
- Snow RW, Rowan KM, Greenwood BM (1987) A trial of permethrin-treated bed nets in the prevention of malaria in Gambian children. Trans R Soc Trop Med Hyg 81: 563-567.
- Quinones ML, Lines J, Thomson MC, Jawara M, Greenwood BM (1998) Permethrin-treated bed nets do not have a mass killing effect on village populations of Anopheles gambiae s.l. in the Gambia. Trans R Soc Trop Med Hyg 92: 373-378.
- Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, et al. (2008) Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am J Trop Med Hyg 79: 12-18.
- Genton B, Hii J, Al-Yaman F, Paru R, Beck HP (1994) The use of untreated bed nets and malaria infection, morbidity and mortality. Annals of Trop Med and Parasitology 8: 263-270.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 13943
- [From(publication date):
July-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9418
- PDF downloads : 4525