Research Article Open Access
Validated Method Development for Estimation of Formoterol Fumarate and Mometasone Furoate in Metered Dose Inhalation Form by High Performance Liquid Chromatography
Katari Srinivasarao1,2*, Vinayk Gorule3, Venkata Reddiah Ch3 and Venkata Krishna A3
1CMJ University, Modrina Mansion, Laitumkhrah, Meghalaya, India
2Manager-AD, IDA, Jeedimetla, Hyderabad, India
3Bombay University, Matunga, Mumbai, India
- *Corresponding Author:
- Katari Srinivasarao
Manager-AD, 22-110, IDA
Jeedimetla, Hyderabad
Andhra Pradesh, CMJ University
Modrina Mansion, Laitumkhrah
Meghalaya, India
Tel: +91-9666882927
E-mail: katari_srinivas@rediffmail.com
Received date: November 09, 2012; Accepted date: November 26, 2012; Published date: December 03, 2012
Citation: Srinivasarao K, Gorule V, Venkata Reddiah Ch, Venkata Krishna A (2012) Validated Method Development for Estimation of Formoterol Fumarate and Mometasone Furoate in Metered Dose Inhalation Form by High Performance Liquid Chromatography. J Anal Bioanal Tech 3:153. doi: 10.4172/2155-9872.1000153
Copyright: © 2012 Srinivasarao K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple, precise, accurate, and stability-indicating method is developed and validated for analysis of formoterol fumarate and mometasone furoate in metered dose inhalation formulations. Separation was achieved on a reversed-phase C18 column (150 mm×4.6 mm i.d., 5 μm) using a mobile phase consisting of Sodium dihydrogen orthophosphate buffer/acetonitrile (50:50, v/v) at a flow rate of 1.0 mL/min and UV detection at 220 nm. This method is validated according to united States Pharmacopia requirements for new methods, which include accuracy, precision, selectivity, robustness, and linearity and range. This method shows enough selectivity, accuracy, precision, and linearity and range to satisfy Federal Drugs Administration/International Conference on Harmonization regulatory requirements. The current method demonstrates good linearity over the range 0.01-0.20 mg/mL of formoterol fumarate with r2=0.999 and 0.40-6.00 mg/mL of mometasone furoate with r2=0.999. The average recovery of the method is 99.9% of formoterol fumarate with a relative standard deviation of 1.94% and 101.5% of mometasone furoate with a relative standard deviation of 0.81%. The degree of reproducibility of the results obtained as a result of small deliberate variations in the method parameters and by changing analytical operators has proven that the method is robust and rugged.
Keywords
Formoterol fumarate; Mometasone furoate; HPLC; Antiasthmatic
Introduction
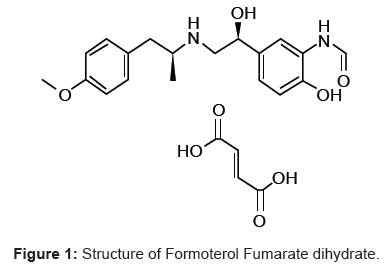
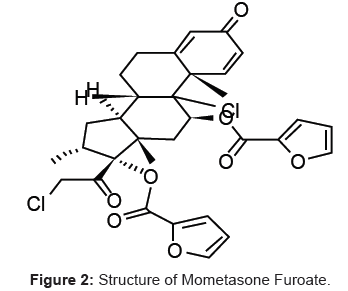
Formoterol fumarate [1,2] and Mometasone furoate [3] is a long-acting beta2-agonist used in the management of asthma and/or chronic obstructive pulmonary disease (COPD). Inhaled formoterol/mometasone works like other beta2-agonists, causing bronchodilatation by relaxing the smooth muscle in the airway so as to treat the exacerbation of asthma. Formoterol fumarate chemical name is N-[2-hydroxy-5-[1-hydroxy-2-[1-(4-methoxyphenyl) propan- 2-ylamino] ethyl] phenyl] (Figure 1) formamide phenylethylamine derivative with one phenolic hydroxyl and one secondary amino group. Mometasone furoate is a corticosteroid used as an anti-inflammatory [3] and chemical name is (11β, 16α)-9, 21-dichloro-11-hydroxy-16- methyl-3, 20-dioxopregna-1, 4-dien-17-yl 2-furoate (Figure 2).
The aim of this study Formoterol fumarate and Mometasone furoate is latest combination of anti asthmatic drugs. It is available in dry powder inhalation and metered dose inhalation form. Both the drugs were individually official in Indian pharmacopoeia [4], United States pharmacopoeia [5] and British pharmacopoeia [6].
The current work presents a reversed phase and stability-indicating method for analysis of formoterol fumarate and mometasone furoate drugs in metered dose inhalation. The method is simple where reversedphase- LC with isocratic elution and UV detection was used. Validation of the method was performed according to the requirements of United States Pharmacopeia for assay determination, which includes accuracy,precision, (repeatability and intermediate precision (ruggedness)), selectivity, robustness, and linearity and range. Additionally, in order to meet the regulatory guidance of the Federal Drug Administration/ International Conference on Harmonization (ICH, Q2 (R1)), [7] formoterol fumarate and mometasone furoate was degraded forcibly in acidic, basic, and thermal at 60°C.
Experimental
Chemicals
Acetonitrile HPLC grade was from Merck specialties private limited (Mumbai, India). Methanol HPLC grade was from Merck specialties private limited (Mumbai, India). Sodium dihydrogen orthophosphate monohydrate (AR Grade), Decane Sulfonic acid sodium salt (AR Grade), Orthophosphoric acid (AR Grade) purchased from Merck specialties private limited (Mumbai, India) and double distilled water were used in analysis. Working standards of pharmaceutical grade formoterol fumarate and mometasone furoate were obtained as generous gifts from Hetero Labs Ltd (Hyderabad, India) and was used as such without further purification.
Apparatus
HPLC system (Shimadzu system, Japan) with a detector (SPD- 20A/20AV Series-Prominence), equipped with a pump (LC-20 AD, 228-45000-32), auto sampler (SIL-10AF), column compartment (CTO-10A VP), and solvent rack (SIL-20) was employed during this study. The LC-solutions software was employed. The chromatographic analysis was performed on C18 column (150 mm×4.6 mm i.d., 5 μm) (Thermo Scientific Corporation, Karlsruhe, Germany).
HPLC chromatographic conditions
Phosphate buffer was prepared by dissolving 1.38 g of Sodium dihydrogen orthophosphate monohydrate and 1.22 g of decane sulphonic acid of sodium salt monohydrate in 1000 mL of water, and adjusting the pH to 3.0 with dilute phosphoric acid solution. Filtered and degassed mixtures of acetonitrile and buffer (different volume fractions) were tested as mobile phases for Formoterol fumarate and mometasone furoate analysis. Different flow rates (0.5, 1.0, and 1.5 mL/ min) were also tested. UV detection was performed at 220 nm and injection volume was 100 μL.
Standard solutions
Stock standard solution of formoterol fumarate and mometasone furoate were prepared by dissolving a quantity of formoterol fumarate and mometasone furoate equivalent to 12.0 mg of formoterol fumarate and 40.0 mg of mometasone furoate in 100 mL of mobile phase to obtain a solution having known concentration of 120.0 mg/mL formoterol fumarate and 400 mg/mL mometasone furoate.
Nominal (working) standard solutions were prepared by diluting 1 mL of formoterol fumarate stock standard solution and 10 mL of mometasone furoate stock standard solution to 50 mL mobile phase to obtain a solution having a known concentration of 2.4 mg/mL formoterol fumarate and 80 mg/mL mometasone furoate.
Transfer 5.0 mL of above solution into a 100 mL volumetric flask dilute to volume with mobile phase. Those solutions having known concentrations of 0.12 mg/mL formoterol fumarate and 4.00 mg/mL mometasone furoate.
Sample solutions
Sample A: A container was taken, is fitted in an actuator and the valve is primed by wasting first two actuations. The container was shaken for five seconds in between each actuation. The container was removed from its actuator and is washed with mobile phase. The valve and valve stem is cleaned internally and externally with an airline fitted with a narrow jet. A disc is placed in clean and dry 100 mL beaker; added about 40 mL of mobile phase. The container was hold in inverted position, shaken for 5 seconds and deliverd one actuation in beaker through the hole provided in the center of the disc. Similarly further nine actuations are delivered in the same beaker with constant shaking for at least five seconds in between each delivery.
The above solution was transferred from beaker into a 100 mL volumetric flask. The beaker and beaker is washed with mobile phase and transferred into the same volumetric flask. The volume is made up to the mark with mobile phase. 5 mL of the above solution is transfered in to 25 mL volumetric flask, dilute to volume with mobile phase and mix.
Sample B: The actuator is washed with mobile phase and dried gently. The container is fitted in actuator & 10 actuations are delivered with constant shaking for at least 5 seconds in between each delivery. The container is removed from actuator and the actuator is washed 3-4 times with 10 mL mobile phase, and is collected in 25 mL volumetric flask and then volume is made up to the mark with mobile phase.
Content of Active Ingredient Delivered per actuation=μg of Sample A μg of Sample B

Results and Discussion
Method development
Preliminary studies involved trying C8 and C18 reversed-phase columns and testing several mobile phase compositions were conducted for the separation of formoterol fumarate and mometasone furoate with good chromatographic parameters (e.g. minimized peak tailing and good theoretical plates). A C18 column (150 mm×4.6 mm i.d., 5 μm) as a stationary phase with a mobile phase of acetonitrile/phosphate buffer pH 3.0 (50:50, v/v) at a flow rate of 1.0 mL/min and detection wavelength of 220 nm afforded the best separation of formoterol fumarate and mometasone furoate.
Method validation
After method development, validation of the current test method for formoterol fumarate and mometasone furoate were performed in accordance with United States Pharmacopeia requirements for assay determination (category-I: analytical methods for quantitation of active ingredients in finished pharmaceutical products) which include accuracy, precision, selectivity, robustness, and linearity and range.
Linearity and range: To evaluate linearity of the method, six calibration standards of formoterol fumarate containing 0.013, 0.025, 0.064, 0.127, 0.157, and 0.193 mg/mL and mometasone furoate containing 0.403, 0.806, 2.016, 4.031, 4.998, and 6.127 mg/mL were analyzed. A plot of peak areas versus formoterol fumarate and mometasone furoate concentrations was linear in the range from 0.013 to 0.193 mg/mL of formoterol fumarate and 0.403 to 6.127 mg/mL with a correlation coefficient of 0.9996 and 0.9999. This result demonstrates linearity of this method over the specified range.
Accuracy and percentage recovery: Accuracy of the method was studied by preparing the placebo of the drug formulation according to the formulation procedure. To the required quantity of placebo, a known quantity of formoterol fumarate and mometasone furoate with the same proportion as in the drug formulation was added to get three concentrations (0.064, 0.127, and 0.193 mg/mL of formoterol fumarate and 2.016, 4.031, and 6.127 mg/mL of mometasone furoate). Results have shown that the recovery of formoterol fumarate and mometasone furoate is within 98.2-100.4%, and 101.4-102.3% the RSD is lower than 1.0% (Table 1).
| Formoterol fumarate concentration (mg/mL) | Accuracy (% recovery) | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Mean | RSD (%) | |
| 0.064 | 98.9 | 98.7 | 98.3 | 98.6 | 0.30 |
| 0.127 | 98.1 | 98.2 | 98.2 | 98.2 | 0.01 |
| 0.193 | 100.5 | 100.2 | 100.5 | 100.4 | 0.17 |
| Mometasone furoate concentration (mg/mL) | Accuracy (% recovery) | ||||
| Sample 1 | Sample 2 | Sample 3 | Mean | RSD (%) | |
| 2.016 | 101.8 | 102.0 | 101.8 | 101.9 | 0.08 |
| 4.031 | 101.4 | 101.3 | 101.3 | 101.4 | 0.07 |
| 6.127 | 102.3 | 102.3 | 102.3 | 102.3 | 0.01 |
Table 1: Accuracy (% recovery) of formoterol fumarate and mometasone furoate in metered dose inhalation formulation at three concentration levels.
Precision
Repeatability: Repeatability of the method was evaluated by calculating the RSD of the peak areas of six replicate injections for the standard concentration (100%) of formoterol fumarate and mometasone furoate, which were found to be 0.37% and 0.09%. Furthermore, the RSD of the peaks areas of the recovery data analyzed in accuracy study (see Section 3.2.2) for each level was calculated, and it was found to be less than 1.0% for both drugs and each level (0.30%, 0.01%, and 0.17% for 50%, 100%, and 150%, for formoterol fumarate and 0.08%, 0.07%, and 0.01% for 50%, 100%, and 150%, for mometasone furoate respectively), as shown in table 1. These results show that the current method for formoterol fumarate and mometasone furoate analysis is repeatable.
Intermediate precision (ruggedness): Intermediate precision (also called ruggedness) of the method was also evaluated by analyzing six samples of formoterol fumarate and mometasone furoate by two analysts in the same laboratory using different HPLC systems. Results of this study showed that the RSD of the percentage of formoterol fumarate and mometasone furoate in formoterol fumarate and mometasone furoate metered dose inhalation for the 12 samples (6 samples from each analyst) were 1.25% and 0.98% indicating a good intermediate precision of the method.
Selectivity (stability indicating evaluation): Selectivity of the method was demonstrated by enhancing degradation of formoterol fumarate and mometasone furoate under liquid and solid stress conditions (acid, base hydrolysis and oxidation, thermal, UV and humidity), to show that formoterol fumarate and mometasone furoate were separated from possible degradation products of formoterol fumarate and mometasone furoate resulted from liquid/solid stress condition. Results have shown that formoterol fumarate and mometasone furoate were stable in hydrogen peroxide solution (it gives no degradation products). Furthermore, it was found that formoterol fumarate and mometasone furoate were stable when standard solution of it were stored at room temperature or in oven at 60°C for one week. On the other hand, formoterol fumarate and mometasone furoate were not stable in acidic and basic solutions; about 40% and 45% of formoterol fumarate and 50% and 40% of mometasone furoate were degraded in hydrochloric acid solution and sodium hydroxide solution, respectively. However, no degradation products were detected.
Robustness: Robustness of the current method was investigated by analyzing three samples of formoterol fumarate and mometasone furoate in metered dose inhalation formulations using the same chromatographic conditions set forth in method development but (a) using flow rate 0.9 and 1.1 mL/min instead of 1.0 mL/min; (b) using column oven temperature 35°C and 45°C instead of 40°C; (c) using buffer pH 2.8 and 3.2 instead of pH 3.0; (d) using acetonitrile/ buffer 52:48 and 48:52 instead of 50:50, v/v. RSD of the percentage of formoterol fumarate and mometasone furoate under these conditions is calculated to be less than 2.0% (Table 2).
| Content of formoterol fumarate assay (%) | |||||
| Parameter | Sample 1 | Sample 2 | Sample 3 | Mean | RSD (%) |
| Flow rate (mL/min) | |||||
| 0.9 | 96.5 | 98.2 | 97.2 | 97.3 | 0.88 |
| 1.0 | 101.2 | 99.9 | 100.2 | 100.4 | 0.68 |
| 1.1 | 98.7 | 99.7 | 99.8 | 99.4 | 0.61 |
| Column temperature (°C) | |||||
| 35°C | 94.2 | 95.8 | 95.2 | 95.1 | 0.85 |
| 40°C | 101.2 | 101.2 | 102.4 | 101.6 | 0.68 |
| 45°C | 97.0 | 98.5 | 98.0 | 97.8 | 0.78 |
| Buffer pH 3.0 | |||||
| 2.8 | 96.4 | 99.1 | 95.8 | 97.1 | 1.81 |
| 3.0 | 99.8 | 102.8 | 99.4 | 100.7 | 1.85 |
| 3.2 | 94.0 | 97.5 | 95.8 | 95.8 | 1.83 |
| Mobile Phase composition (v/v) | |||||
| 48:52 | 99.4 | 102.5 | 101.3 | 101.1 | 1.55 |
| 50:50 | 99.0 | 99.1 | 99.6 | 99.2 | 0.32 |
| 52:48 | 99.2 | 102.5 | 102.1 | 101.3 | 1.78 |
| Content of mometasone furoate assay (%) | |||||
| Parameter | Sample 1 | Sample 2 | Sample 3 | Mean | RSD (%) |
| Flow rate (mL/min) | |||||
| 0.9 | 102.6 | 102.4 | 101.8 | 102.3 | 0.41 |
| 1.0 | 103.7 | 103.1 | 103.4 | 103.4 | 0.29 |
| 1.1 | 100.7 | 103.0 | 104.6 | 102.8 | 1.91 |
| Column temperature (°C) | |||||
| 35°C | 102.8 | 102.9 | 102.4 | 102.7 | 0.26 |
| 40°C | 103.0 | 103.0 | 102.6 | 102.9 | 0.22 |
| 45°C | 101.0 | 101.2 | 100.9 | 101.0 | 0.15 |
| Buffer pH 3.0 | |||||
| 2.8 | 102.0 | 102.5 | 101.7 | 102.1 | 0.40 |
| 3.0 | 101.5 | 101.7 | 101.2 | 101.5 | 0.25 |
| 3.2 | 101.4 | 100.1 | 102.0 | 101.2 | 0.96 |
| Mobile Phase composition (v/v) | |||||
| 48:52 | 100.8 | 101.8 | 105.7 | 101.8 | 0.93 |
| 50:50 | 101.1 | 101.1 | 101.4 | 101.2 | 0.17 |
| 52:48 | 101.4 | 103.0 | 105.3 | 103.2 | 1.90 |
Table 2: Robustness testing of the formoterol fumarate and mometasone furoate.
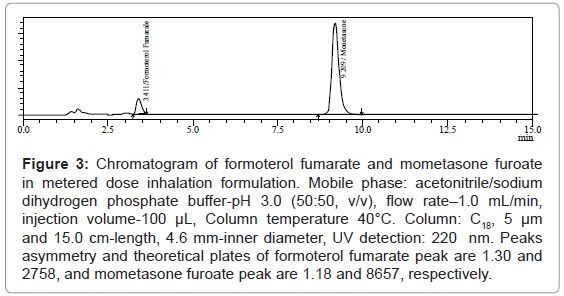
After successful development and validation of this method, it was employed for analysis of formoterol fumarate and mometasone furoate in metered dose inhalation formulations as shown in (Figure 3).
Figure 3: Chromatogram of formoterol fumarate and mometasone furoate in metered dose inhalation formulation. Mobile phase: acetonitrile/sodium dihydrogen phosphate buffer-pH 3.0 (50:50, v/v), flow rate–1.0 mL/min, injection volume-100 μL, Column temperature 40°C. Column: C18, 5 μm and 15.0 cm-length, 4.6 mm-inner diameter, UV detection: 220 nm. Peaks asymmetry and theoretical plates of formoterol fumarate peak are 1.30 and 2758, and mometasone furoate peak are 1.18 and 8657, respectively.
Conclusion
A simple, accurate, precise, and stability-indicating HPLC method was developed and validated for the routine analysis of formoterol fumarate and mometasone furoate in metered dose inhalation formulations. The results of stress testing undertaken according to the International Conference on Harmonization guidelines reveal that the method is selective and stability-indicating.
Acknowledgements
We would like to thank gratefully Hetero labs limited (Hyderabad, India) for the gift sample of pure formoterol fumarate and mometasone furoate. Thanks also extended to Dr. Vinayk Gorule, Bombay University for providing necessary facilities and constant support.
References
- O’Neil MJ (2006) The Merck Index an encyclopedia of Chemicals, Drugs and Biologicals. (14thedn), Merck & Co.Inc, 4244.
- Sweetman SC (2007) Martindale the Complete Drug Reference. (35thedn), Pharmaceutical press, London, 925.
- Medscape.
- Indian Pharmacopoeia, Ministry of Health and Family Welfare, Government of India, New Delhi, 2010. (Addendum 2012); 1382, 2934, 1386.
- United States Pharmacopoeia (USP 35, 2012); 3279, 3943, 3261.
- British Pharmacopoeia (2012) London, Medicines and Health care products regulatory agency (MHRA); 966, 1492, 956.
- ICH, Q2 (R1), Harmonized tripartite guideline, Validation of analytical procedures: text and methodology, International Conference on Harmonization ICH, Geneva, Nov 2005.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16168
- [From(publication date):
December-2012 - Apr 08, 2025] - Breakdown by view type
- HTML page views : 11287
- PDF downloads : 4881