Review Article Open Access
Vaccine Development for Biothreat Alphaviruses
Kevin B Spurgers and Pamela J Glass*
United States Army Medical Research Institute of Infectious Diseases, Frederick, MD 21702, USA
- *Corresponding Author:
- Pamela J Glass
United States Army Medical Research Institute of Infectious Diseases
1425 Porter Street,Fort Detrick, Frederick, Maryland 21702
Tel: 301-619-4742
E-mail: Pamela.glass@amedd.army.mil
Received Date: June 14, 2010; Accepted Date: July 13, 2011; Published Date: September 25, 2011
Citation: Spurgers KB, Glass PJ (2011) Vaccine Development for Biothreat Alpha viruses. J Bioterr Biodef S1:001. doi: 10.4172/2157-2526.S1-001
Copyright: © 2011 Spurgers KB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
The majority of alphaviruses are non-pathogenic to humans. However, select alphaviruses can cause severe disease in humans during the course of naturally occurring epizootic outbreaks, or accidental infection of laboratory personnel. Natural infections occur through the bite of an infected mosquito. However, pathogenic alphaviruses, including Venezuelan, eastern, and western equine encephalitis viruses, have proven to be highly infectious via the aerosol route. Given this aerosol infectivity, ease of production of high-titer virus, and low infectious dose, these alphaviruses are recognized as candidates for use as biological weapons, and are classified as category B pathogens by the Centers for Disease Control and Prevention and The National Institutes of Health. There are currently no licensed vaccines to prevent alphavirus infections. Such a vaccine could protect geographically defined human populations during an epizootic, and enhance national security by serving as a deterrent to the use of these viruses as biological weapons. To address this critical need, several strategies are being pursued to develop safe, effective, and ultimately licensed vaccines for use in humans.
Keywords
Alphavirus; Vaccine; Attenuated; Venezuelan equine encephalitis; Eastern equine encephalitis; Western equine encephalitis; Adenovirus; Furin; Chimeric; Subunit; DNA; Virus-like replicon particles; Inactivated
Introduction
Venezuelan (VEEV), eastern (EEEV), and western equine encephalitis (WEEV) viruses, members of the genus Alphavirus in the family Togaviridae, are causative agents of debilitative, acute, and sometimes fatal encephalitis in North, Central, and South America [1]. These viruses are maintained in nature in a zoonotic cycle between susceptible nonhuman vertebrate hosts, and hematophagous mosquito vectors. Natural human cases are rare, and occur via the bite of an infected mosquito. Since the discovery of these viruses, several epizootic outbreaks, infecting human and equid livestock populations, have been recognized. Additionally, these viruses pose a threat to public health, and military personnel because of their potential use as bioweapons [2]. This threat is based on virus characteristics favorable to weaponization, and a known history of weaponization. First, these viruses have been proven to be highly infectious by the aerosol route. They are also easy to produce at high titer, have a low infectious dose, and can be lyophilized. VEEV was tested as a biowarfare agent during the U.S. offensive program in the 1950's and 1960's, and may have been weaponized by the former Soviet Union [3,4]. Because of the potential for weaponization, VEEV, EEEV, and WEEV are classified as category B pathogens by the Centers for Disease Control and Prevention (CDC), and the National Institutes of Health (NIH). Veterinary vaccines utilizing inactivated alphavirus preparations are available and in routine use to control infection in endemic areas [5]. Unlicensed, investigational vaccines for VEEV, EEEV, and WEEV are also in use to protect at-risk laboratory personnel [6-8]. There are currently no vaccines licensed for general use in the U.S. for prevention or treatment of alphavirus infections.
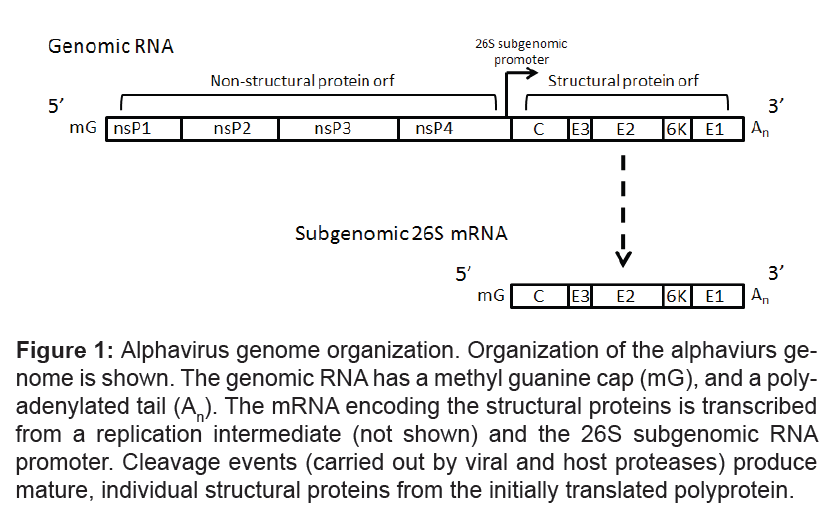
Alphavirus virions are small, spherical particles ~ 70 nm in diameter [1]. The viral nucleocapsid core is surrounded by a host-derived lipid membrane in which 80 protein spikes composed of trimers of E1/ E2 heterodimers are embedded. The nucleocapsid consists of the capsid protein (C) surrounding the single-strand, positive sense, ~11 kb RNA genome. The genomic RNA is capped, has a polyadenylated tail, and is immediately translated upon entry into the cell cytoplasm. The 5' region of the genome encodes four nonstructural proteins (responsible for viral transcription and replication), while the 3' region codes for five structural proteins (Figure 1). The structural genes are initially expressed as a polyprotein from a 26S subgenomic RNA (Figure 1). Cleavage events (by furin and signalase) produce the mature structural proteins, including C, E1, and E2, as well as E3 and 6K [1,9,10]. The E2 glycoprotein is thought to be involved in receptor binding [11,12]. The E1 glycoprotein has a role in endosomal membrane fusion, and release of the nucleocapsid into the cytoplasm [13,14]. In response to infection, most neutralizing antibodies are produced targeting the E2 protein. Given that many studies have demonstrated that a neutralizing antibody response correlates with protection against a subcutaneous challenge, E2 is the most common antigen used in vaccine efforts to combat alphavirus infections. Although, neutralizing antibodies against E1 protein are rare, E1 alone has been successfully used as a vaccine antigen capable of protecting against lethal challenge [15,16].
Figure 1: Alphavirus genome organization. Organization of the alphaviurs genome is shown. The genomic RNA has a methyl guanine cap (mG), and a polyadenylated tail (An). The mRNA encoding the structural proteins is transcribed from a replication intermediate (not shown) and the 26S subgenomic RNA promoter. Cleavage events (carried out by viral and host proteases) produce mature, individual structural proteins from the initially translated polyprotein.
VEEV represents a complex of viruses previously classified as subtypes I-VI (Table 1). Recent taxonomic changes have classified only the subtype I viruses as VEEV [17]. VEEV subtype varieties IAB and IC have been associated with major outbreaks involving hundreds of thousands of equine and human cases [18]. VEEV subtypes ID, IE, and IF are enzootic, equine avirulent strains not associated with major epizootics or epidemics, although they do occasionally cause humans illness, which can be fatal [19,20]. Subtypes II-VI are now classified as distinct species within the Alphavirus genus. In many cases, immunity to one species or subtype/strain does not protect against a heterologous strain. This is true for the currently available investigational VEEV vaccines which may not protect against heterologous subtypes/strains [7,21,22].
| Complex | Species | Subtype | Strain(s) |
|---|---|---|---|
| VEEV | VEEV | IAB | Trinidad (TrD), TC-83 |
| VEEV | IC | SH3, P676 | |
| VEEV | ID | ZPC738, 3880 | |
| VEEV | IE | 68U201, Mena II | |
| VEEV | IF | 75V3531 | |
| Everglades virus | (II) | Fe3-7c | |
| Mucambo virus | (IIIA) | ||
| Tonate virus | (IIIB) | ||
| Pixuna virus | (IV) | BeAr35645 | |
| Cassabou virus | (V) | ||
| Rio Negro virus | (VI) | ||
| EEEV | EEEV NA | Lineage I | FL93-939 |
| EEEV SA | Lineage II-IV | BeAr436087 | |
| WEEV | WEEV | CBA87 | |
| WEEV | ON41-McMillan | ||
| WEEV | Fleming | ||
| WEEV | CO92-1356 | ||
| WEEV | 71V-1658 | ||
| Highlands J virus | |||
| Fort Morgan virus | |||
| Aura virus | |||
| Sindbis virus | |||
| Whataroa virus |
Table 1: Partial summary of alphavirus strains and subtypes.
The EEEV complex consists of two species, North and South American, which are further divided into four distinct genetic lineages [23]. Lineage I is found in North America and the Caribbean (EEEV NA); and lineages II-IV that are found in Central and South America (EEEV SA). Human disease is associated with the lineage I viruses.
The WEEV complex consists of six species, WEEV, Sindbis virus, Highlands J virus, Fort Morgan virus, Aura virus, and Whataroa virus. The Sindbis group consists of five genotypes found exclusively in Old World distributions [24]. These viruses cause a relatively mild illness with symptoms including fever, rash, and arthralgia. The remaining members of the WEEV complex are New World viruses. There are a number of WEEV subtypes, some of which are antigenically distinct, found throughout North and South America [25,26]. A number of WEEV strains have been associated with disease in humans and horses, although a majority of the cases are either asymptomatic or present as a febrile illness.
Investigational vaccines for VEEV include TC-83 and C-84. TC-83 is a live-attenuated virus generated by serial passage of VEEV Trinidad (TrD) strain in guinea pig heart cells [27]. This vaccine is immunogenic and produces a neutralizing antibody response in approximately 80% of human recipients. However, approximately 40% of vaccinated individuals develop moderate flu-like symptoms, including fever, headache, and malaise. Although this vaccine strain of VEEV can produce longlasting immunity, safety concerns remain. In horses, TC-83 vaccination can produce significant viremia [28]. The virus also causes illness or death in certain mouse strains after intracranial (i.c.), or subcutaneous (s.c.) inoculation [29]. C-84 is a formalin-inactivated preparation of TC-83, which is administered to at-risk individuals who fail to seroconvert after TC-83 vaccination, and those whose titer wanes over time [30]. This vaccine strain is safer than TC-83, but produces reduced neutralizing antibody titers and less durable immune responses. Formalininactivated virus vaccines for EEEV and WEEV are also in use at the U.S. Army Special Immunizations Program to protect at-risk laboratory personnel [6]. The properties of these vaccines are similar to C-84, in that they are poorly immunogenic, require frequent boosting; and it is not clear if they would protect individuals from an aerosol challenge.
A critical need exists to produce safe, effective, and ultimately licensed vaccines for the prevention of VEEV, EEEV, and WEEV infections. For such a vaccine to be effective in a biodefense scenario, it ideally will protect individuals from aerosol exposure to VEEV, EEEV, or WEEV. As such, most vaccine studies now measure efficacy against aerosol virus challenge in various animal models of infection. This is accomplished by testing exposure to artificial aerosols, or infection by the intranasal (i.n.) route. The most appropriate and predictive correlate of protection for alphavirus vaccines is still ill-defined. It is unclear if an antigen specific antibody response (including neutralizing antibodies), or a cell-mediated response, or a combination of both, is critical for a successful vaccination against aerosol VEEV, EEEV, or WEEV challenge. A large number of vaccine development strategies are currently being employed to produce a vaccine that is more immunogenic, more efficacious, and safer than current investigational vaccines for alphaviruses (Figure 2, Table 2). This review summarizes these efforts.
Figure 2: Vaccine platforms used for the development of anti-alphavirus vaccines. Chimeric virus vaccine candidates typically harbor a recombinant RNA genome coding for the non-structural proteins of SINV, and the structural proteins for VEEV, EEEV, or WEEV. Rationally designed, recombinant, attenuated vaccines feature a deleted furin cleavage site between E3 and E2 coding sequences. This results in PE2 (E2 precursor protein) being expressed on the virion surface, along with virus attenuation. Viral replicon particles (VRPs) are non-replicating, but infectious particles, engineered to express a protein antigen (ag) of interest upon infection. Genome replication and gene expression are carried out the by the nonstructural proteins (nsP 1-4). Viruses of various types, including adenoviruses (Ad) have been engineered to express a vaccine antigen upon transduction of target cells. DNA vaccines are typically plasmids that express a vaccine antigen upon entry into target cells. Finally, peptides from viral proteins or whole viral proteins can be administered to elicit a protective immune response.
| Challenge Virus | Vaccine platform | Test Species | References |
|---|---|---|---|
| VEEV | Adenovirus | Mice (BALB/c) | [65,66,68,85] |
| Equine Herpesvirus type I | Mice (NIH Swiss) | [71] | |
| Vaccinia virus | Mice (NIH Swiss, A/J, C3H, BALB/c) | [77-80] | |
| DNA | Mice (BALB/c), rabbit, NHP | [81-85] | |
| SINV/VEEV Chimeras | Mice (NIH Swiss), Hamster | [31,32] | |
| VRP | Mice (BALB/c), NHP | P Glass, D Reed, manuscript in preparation | |
| Attenuated (furin cleavage site mutant) | Mice (BALB/c, CH3, C57BL/6, CD-1), hamster, Horse, NHP | [42-48] | |
| Attenuated and Inactivated | Mice (BALB/c, CD-1) | [49-52] | |
| Subunit | Mice (BALB/c, NIH Swiss) | [89,90] | |
| WEEV | Adenovirus | Mice (BALB/c) | [16,69,70] |
| SINV/WEEV Chimera | Mice (NIH Swiss) | [33,34] | |
| DNA | Mice (BALB/c) | [15,86] | |
| Attenuated (furin cleavage site mutant) | Chickens | [41] | |
| Subunit | Mice (BALB/c) | [87,88] | |
| EEEV | SINV/EEEV Chimeras | Mice (NIH Swiss) | [35,36] |
| Attenuated and Inactivated | Mice (BALB/c) | P Glass, personal communication |
Table 2: Summary of select alphavirus vaccine studies.
Live attenuated virus vaccines
Chimeric Vaccines: Construction of virus chimeras often produces replication-competent, but highly attenuated viruses that are attractive vaccine candidates. Chimeric viruses have been developed and tested as vaccine candidates for the prevention of infection with VEEV, EEEV, and WEEV. The most common strategy uses Sindbis virus (SINV), typically not pathogenic to humans, as a vector to express the structural genes of VEEV, EEEV, or WEEV. Such chimeric viruses are attenuated and can protect mice from lethal alphavirus infection. For example, a chimeric virus (SIN-83) containing the nonstructural genes of SINV, and the full structural gene region of VEEV TC-83 protects mice from lethal VEEV challenge [31]. Although lower than that seen with TC-83 immunization, SIN-83 produced a neutralizing antibody response to VEEV TC-83. To examine vaccine efficacy, NIH Swiss mice were vaccinated with one dose of either VEEV TC-83 or SIN-83 by the s.c. route. Four weeks later, mice were challenged s.c. with VEEV strains ZPC738 or SH3. All mice immunized with either TC-83 or SIN-83 survived challenge. Although SIN-83 grows well in cell culture, it is highly attenuated. Intracranial injection of SIN-83 (2x106 plaque forming units, pfu) produced no mortality in suckling mice. Chimeric viruses using other SINV and/or VEEV strains have also been examined [32]. These included SINV chimeras using structural genes from virulent VEEV strains TrD and ZPC738. Additionally, a more virulent strain of SINV was utilized with VEEV TrD. These chimeras showed intermediate attenuation when tested in suckling mice compared to SIN-83, but were more efficacious than SIN-83 in a mouse challenge model. Efficacy was also demonstrated in Syrian golden hamsters after s.c. challenge with VEEV ZPC738. Overall, these SINV/VEEV chimeras were found to be safer than TC-83, and more efficacious than the original SIN-83 chimera in s.c. challenge models of infection.
Atasheva et al., reported the use of three recombinant viruses as vaccines against WEEV infection [33]. These chimeras are based on the SINV backbone, and express the SINV nonstructural genes required for virus replication. The recombinant viruses are engineered to express the structural genes of WEEV (strain CO92-1356 or ON41-McMillan). SINV/CO92 was safe in adult mice, but was poorly immunogenic, and provided 100% protection against lethal WEEV infection only at the highest dose of vaccine tested. Two additional SINV/WEEV chimeras had greatly improved immunogenicity, as measured by a robust neutralizing antibody response [33]. One of these proved safe in adult mice, and provided 100% protection against i.n. challenge with WEEV after one vaccination dose. Despite these promising results, the chimeras were highly pathogenic when administered to suckling mice, leaving some concerns regarding safety. Environmental safety of these chimeras has been addressed by examining the ability of the viruses to infect, and be spread by, mosquitoes [34]. By this measure, the viruses appear to be safe, and unlikely to be reintroduced into a natural transmission cycle.
A similar approach generated chimeras between SINV and EEEV FL93-939 or EEEV BeAr436087 (termed SIN/NAEEEV and SIN/ SAEEEV, respectively) [35]. Neither chimera produced disease or death in 8-week old NIH Swiss mice. SINV/NAEEEV was more immunogenic and produced a good neutralizing antibody response against homologous EEEV. For both chimeras, the neutralizing antibody response was slightly cross-reactive to heterologous EEEV. Importantly, both chimeras provided complete or near complete protection against intraperitoneal (i.p.) challenge with EEEV FL93-939, at all doses of vaccine tested. As with the SINV/WEEV chimeras, neurovirulence was observed in suckling mice. Additionally, dissemination potential of the chimeric virus was noted in Ae. sollicitans [36].
Furin cleavage site mutant vaccines: The E2 protein of alphaviruses begins its transit through the endoplasmic reticulum and golgi as PE2, a precursor protein consisting of E3 and E2. In the trans-Golgi network, or a post-Golgi compartment, E3 is cleaved from PE2 by furin, producing mature E2 [10,37-40]. Alphaviruses incorporating PE2 into mature virions can be viable and infectious, but are generally attenuated in animal models of infection, and grow poorly in mosquito cells. These observations have led to the rational design of VEEV, EEEV, and WEEV vaccine candidates with furin cleavage site deletions, and associated secondary resuscitating mutations [41,42].
Site-directed mutagenesis was applied to the virulent VEEV TrD strain, to produce engineered virus with deletion or mutation of the furin cleavage site of PE2 [42]. Secondary mutations in either E2 or E1 allow the production of viable, infectious virions incorporating PE2 into the virus membrane. One furin cleavage mutant that has been well characterized is V3526. This recombinant virus harbors a deletion of the furin cleavage site and a secondary mutation at codon 253 in E1. VEEV V3526 demonstrates reduced growth in C6/36 mosquito cells. Growth of this virus is also slower in mammalian BHK cells, but final titers of V3526 can equal those of wild type VEEV TrD (clone V3000) virus. V3526 produces no signs of disease, or death, when administered to adult CD-1 mice by the i.n. or s.c. routes. These immunized mice were also completely protected against lethal i.n. challenge with VEEV TrD V3000 virus [42]. Since this initial characterization, V3526 has proven to be a highly efficacious and safe vaccine in several animal models, and was transitioned into clinical development [43-48]. However, V3526 vaccination induced unacceptable clinical signs in humans during phase I clinical trials and the vaccine was placed on hold (Parker MD, unpublished data). At this point, the decision was made by the sponsor of these studies not to pursue live virus vaccines for alphaviruses. An observation that was noted based on the outcome of the clinical trial was that humans appear to be more sensitive to VEEV infection than the current nonhuman primate (NHP) models.
Furin cleavage site mutants also exist for WEEV [41]. Attenuation was demonstrated for two of these recombinant viruses (WE2102 and WE2130) by reduced replication in mosquitoes. These vaccine strains also prevented viremia in chickens after challenge with virulent WEEV [41].
Second-Generation inactivated vaccines
Following the cessation of the live-attenuated vaccine program, studies were conducted to examine inactivated preparations of V3526 as vaccine candidates. V3526 preparations inactivated with 1,5 iodonaphthyl azide (INA), formalin, and gamma irradiation, have been investigated for immune response and efficacy in a mouse model of VEEV infection [49-52]. Methods for complete inactivation of V3526 virus stocks via formalin treatment or gamma irradiation were evaluated and optimized [49]. A detailed dosage and schedule study, with and without adjuvant, was completed to evaluate the immunogenicity and efficacy of gamma irradiated V3526 (gV3526) [50]. The adjuvants tested in this study were CpG, Alhydrogel™ (AlOH), and CpG+AlOH. BALB/c mice immunized s.c., or intramuscular (i.m.; low dose), with or without adjuvant, were significantly protected against s.c. challenge with VEEV TrD. Under these conditions, protection was absent or poor against aerosol exposure to VEEV. However, increasing the dose of gV3526 administered i.m. with adjuvant (CpG alone, or CpG+ AlOH) resulted in 70-90% protection against aerosolized VEEV TrD.
A similar study was carried out with formalin-inactivated V3526 (fV3526) [51]. The same adjuvants mentioned above were tested as well as one additional adjuvant Viprovex. Adjuvant was not required to achieve 100% serconversion in BALB/c mice after one or two vaccinations, s.c. or i.m.. Neutralizing antibody titers after fV3526 vaccination approached those achieved with C84 vaccination. Adjuvant was also not required for fV3526 vaccination (s.c.) to protect 100% of mice from s.c. VEEV TrD challenge. However, fV3526 alone offered poor protection against aerosol challenge. Inclusion of AlOH adjuvant increased survival to 80% (compared to 70% survival for C84 vaccination). Similar results were obtained when vaccinating by the i.m. route. One notable exception was the increased protection against aerosol challenge afforded by fV3526 + CpG adjuvant administered i.m. compared to s.c. vaccination. VEEV-specific serum and neutralizing antibodies were produced to both fV3526 and gV3526 regardless of vaccine dose, schedule or adjuvant. However, a positive antibody response, total serum or neutralizing, could not be correlated with protection against aerosol challenge. In both the gV3526 and fV3526 studies, mice were vaccinated with extremely low doses. It is likely that further increases in dose of these vaccine candidates could achieve complete protection against an aerosol challenge.
The photoactive compound 1,5 iodonaphthyl azide (INA) sequesters in lipid membranes and, with ultraviolet irradiation, covalently binds to lipids and proteins in the lipid bilayer. This reaction inactivates integral membrane proteins while preserving extracellular epitopes on those proteins. This compound has been shown to completely inactivate several viruses including ebolavirus, HIV-1, VEEV (TrD, clone V3000), and V3526 [52-54]. Interestingly, Sharma et al., observed that RNA isolated from INA-treated VEEV or V3526 was noninfectious, and did not produce new virions or cell death when transfected into BHK cells [52]. This raises the possibility that INA inactivates virions by two independent mechanisms. Vaccination with INA-treated VEEV offers partial and dose-dependent protection against s.c. challenge with VEEV TrD. This protection is enhanced with adjuvant. Additional studies are required to investigate the efficacy of INA-inactivated V3526 against aerosol VEEV challenge.
While inactivated vaccines were developed as first-generation vaccines, many advances in techniques and methodologies have warranted reexamination of this strategy for production of secondgeneration alphavirus vaccines. Previous studies indicate that inactivated vaccines are safe and effective. Work is now underway with inactivated V3526 to examine increased vaccine dose and the use of adjuvants. Similar strategies for the production of inactivated EEEV and WEEV vaccines are also being investigated (Glass, P., personal communication).
Viral replicon particle vaccines
Viral replicon particles (VRPs), based on alphavirus vectors, are a promising vaccine platform that has proven safe and efficacious in a variety of animal models [55]. VRPs consist of a self-replicating RNA genome (replicon), which expresses the alphavirus nonstructural genes (producing the proteins required for viral transcription and genome replication), along with a gene(s) of interest. The heterologous gene(s) take the place of the viral structural genes in the replicon RNA. VRPs are generated by transfection of target cells with the replicon RNA along with helper RNAs which express the viral structural genes. The helper RNAs do not contain signal sequences required for packaging into new viral particles, therefore, only the replicon RNA is packaged into the VRP. The resulting VRPs infect new cells and express the inserted gene of interest, but do not generate new VRPs.
VRP-based vaccines have been used to successfully protect animals from challenge with simian-human immunodeficiency virus (SHIV) [56], measles virus [57], and ebolavirus [58]. A similar vaccination approach was efficacious in mouse and NHP animal models of alphavirus infection (Glass, P. and Reed, D., manuscripts in preparation). Specifically, a replicon containing the VEEV nonstructural proteins was engineered to express the envelope glycoproteins of VEEV, EEEV, or WEEV. Mice vaccinated with the VRPs exhibited complete protection from lethal aerosol challenge with VEEV, EEEV, or WEEV (Glass, P. manuscript in preparation). Vaccination with the trivalent VRP vaccine also significantly improved clinical parameters in NHPs challenged with aerosolized VEEV (Reed, D., manuscript in preparation). In each of these experiments, vaccine was administered on day 0, with a boost on day 28. The one shortcoming was that very large doses of VRP were required to protect NHPs. Ongoing studies are investigating the use of adjuvants to decrease the requisite dose of VRP needed for protection. Given successful protection against several viral infections, VRPs remain an attractive vaccine candidate for advanced development.
Virus-Vectored vaccines
Viral vectors of several types, engineered to express a transgene/protein antigen of interest upon transduction of target cells, have been widely studied and utilized as vaccines [59]. In particular, replication-incompetent adenoviruses (Ad) are being used as pre-clinical and clinical tools to combat infectious disease, cancer, and Alzheimer's disease [60]. Adenovirus-based vaccines to prevent various viral infections, including HIV-1, influenza A, dengue virus, and Japanese encephalitis virus have been described [61-64]. These vaccines can produce potent antigen-specific antibody and T-cell immune responses. Adenovirus- vectored vaccines administered i.n. produce mucosal immunity, which may be an important factor for preventing disease after aerosol exposure to viral pathogens. Other viruses used as vaccine vectors include vaccinia virus, Sendai virus, and lentivirus, among others.
Adenovirus-Vectored vaccines: The adenovirus vaccine platform has been used successfully to immunize mice against lethal VEEV infection. Phillpotts et al., utilized an adenovirus vector expressing the E3-E2-6K portion of the subgenomic region of VEEV TC-83 [65]. This recombinant adenovirus expresses VEEV E2 protein upon infection of target cells. Three sequence changes were engineered into the E2 gene sequence of this construct, to match the sequence of E2 in the VEEV Trd strain (vaccine designated RAd/VEEV#3). This vaccine candidate provided significant protection against challenge doses of 640 LD50 or less; however it did not protect BALB/c mice challenged with a high dose (8460 LD50) of aerosolized VEEV TrD. This vaccine candidate did provide protection against heterologous strains of VEEV however. Partial or complete protection was achieved when mice were challenged with ~100 LD50 of VEEV strains from five other serogroups. With this vaccine, an antigen-specific antibody response was achieved. However, sera from immunized mice were unable to neutralize VEEV TrD. The antibody response to VEEV antigen after immunization with RAd/ VEEV#3 was not improved with co-administration of CpG oligodeoxynucleotides as adjuvant [66]. Although more survivors were observed in the vaccine plus adjuvant group (versus vaccine alone) after challenge with a high dose of VEEV TrD, this apparent increase in protection was not statistically significant. Likewise, plasmid or Ad-directed expression of interferon alpha as an adjuvant to RAd/VEEV#3, did not improve the immunogenicity of the RAd/VEEV#3 vaccine [67]. Significant improvements to this adenovirus-vectored VEEV vaccine were achieved with gene optimization procedures [68]. The RAd/VEEV#3 vaccine construct was altered to optimize codon usage and remove undesirable RNA motifs. These alterations increased E2 expression in transduced cells and increased the VEEV-specific antibody response in mice. The optimized vaccine construct also significantly improved survival after VEEV challenge when compared to the parental RAd/ VEEV#3 vaccine [68].
Adenovirus vectors have also been investigated as a vaccine platform to prevent WEEV infections. Wu et al., utilized a replication defective adenovirus vector containing the subgenomic coding region (E3-E2-6K-E1) of WEEV strain 71V-1658 [69]. Upon infection of cells with this construct (Ad5-WEEV) WEEV E1 and E2 proteins are expressed. BALB/c mice administered two doses of Ad5-WEEV i.m. produce a modest neutralizing antibody response and are protected against i.n. homologous WEEV challenge [69]. In a follow-up study, the authors demonstrate both rapid and long-lasting cross-protection against WEEV challenge with a single dose of vaccine. A single i.m. administration of Ad5-WEEV protected mice from the 71V-1658, CBA87, and Fleming strains of WEEV at one week, or 13 weeks, after immunization [70]. Swayze et al., also constructed and tested an Ad5 vector expressing only WEEV E1 protein (Ad5-E1) [16]. A single i.m. administration of this vaccine construct also completely protected mice against intranasal challenge with WEEV strains 71V-1658 and CBA87. At the time of WEEV challenge, one week after vaccination, a T-cell response was detected in the absence of a humoral immune response. Further studies are necessary to determine if this finding is reflective of a necessary protective response, or due to assay sensitivity. Regardless, this study suggests that WEEV E1 protein alone may be a sufficient and effective vaccine antigen. These studies show that the adenovirus platform is efficacious; however it is still under debate how pre-existing immunity to adenoviruses will affect the widespread utility of this strategy.
Equine herpesvirus-vectored vaccine: As an alternative to adenovirus- based vaccines, a VEEV vaccine has been tested that utilizes equine herpesvirus type I (EHV-1) as a vector, to express VEEV structural proteins, and protect against lethal challenge [71]. A possible advantage of this vaccine platform is the absence of anti-vector immunity in humans. Like adenoviruses, EHV-1 has broad tissue tropism and can accommodate large amounts of exogenous DNA. A replicationcompetent recombinant EHV-1 virus expressing the E3-E2-6K-E1 portion of the VEEV TC-83 genome was constructed (vaccine designated rH_VEEV) [71]. NIH Swiss mice received two s.c. vaccinations with various doses of rH_VEEV, and were then challenged s.c. with 1000 LD50 of VEEV subtype ID strain ZPC738 four weeks after initial vaccination. The highest dose of rH_VEEV provided complete protection in this lethal mouse model. A VEEV-specific antibody response was achieved after the second vaccination, but neutralizing activity was not detected. Low levels of IgG1 and total IgG antibodies, in the absence of IgG2a, were detected suggesting possible roles for cytotoxic T lymphocytes or antibody-dependent cytotoxicity in the protection against VEEV. A number of studies have reported the lack of correlation of protection with neutralizing antibody responses [50,51,72,73] and others have provided evidence of T cell involvement in the immune response against alphaviruses [74-76]. Further studies are necessary to determine the protective immune response following vaccination with this vaccine candidate.
Vaccinia virus-vectored vaccines: Recombinant vaccinia virus has also been used as a vaccine vector to express the structural proteins of VEEV. While these vaccines are efficacious in mice under certain conditions, they are generally less effective than TC-83, and fail to offer full protection against aerosol VEEV exposure. VACC/TC-5A is a recombinant vaccinia virus engineered to express the structural genes of VEEV TC-83 [77]. A/J mice were immunized by intradermal (i.d.) tail scarification. Although present, the vaccine-induced antibody response to VEEV was less than that seen with TC-83 vaccination. Neutralizing antibodies were present but variable, even at the highest dose of vaccine. However, this response was durable; neutralizing antibodies could be detected in some mice up to 16 months after vaccination [78]. Additionally, TC-5A vaccination produced a T-cell response against both the vaccinia vector and TC-83 VEEV virus [78]. VACC/TC-5A effectively protected mice challenged i.p. with VEEV types IAB, IC, ID, and II [77]. However, this vaccine was not able to protect against i.n. TrD challenge tested in A/J, C3H, and NIH Swiss mice. TC-83 vaccination offered complete or near complete protection from aerosol exposure in each mouse strain.
Bennett et al., also investigated a vaccinia virus vector expressing the 26S subgenomic region of VEEV TC-83 [79]. Additionally, the parental vaccine construct (WR100), was altered in an effort to increase VEEV protein expression and immunogenicity. WR100 was engineered to contain a synthetic promoter in front of the VEEV coding sequences, and to introduce a sequence change in E2 to match the sequence of VEEV TrD. VEEV protein expression was increased from this construct (WR103), compared to WR100. Still, the antibody response to WR103 vaccination was low when compared to TC-83, and neutralizing antibodies were not present. Although an improvement over WR100, WR103 offered only partial protection from s.c. challenge with VEEV TrD to BALB/c mice vaccinated by the i.m. route. Vaccinia virus-based vaccines, expressing only portions of the 26S subgenomic structural gene region (expressing E2 only, or E1 only) also protect BALB/c mice against peripheral challenge with VEEV TrD, but fail to completely protect against aerosol challenge [80].
DNA Vaccines
As a vaccine platform, DNA offers several advantages over other strategies. These include the absence of pre-existing vector immunity, as well as ease and low cost of production. Because of the inherent stability of DNA, transport and long-term storage are not problematic. DNA vaccines have proven safe and effective in preclinical animal models and clinical trials. Yet, immunogenicity is often less than that produced by other vaccine platforms, including live-attenuated, inactivated, or viral-vectored vaccines. DNA vaccines are typically plasmids that express a vaccine antigen of interest when delivered into target cells. These expressed proteins are then processed and presented to the immune system to elicit a protective response. Delivery methods include direct i.m. or i.d. injection of naked DNA, particle-mediated epidermal delivery (PMED, i.e. gene gun), or i.m. electroporation (i.m. EP).
DNA vaccination has shown promise in preclinical animal models as a strategy to prevent VEEV disease. A plasmid DNA vaccine expressing the 26S sub-genomic region (C-E3-E2-6K-E1) of VEEV TrD was administered to mice by PMED. This strategy protected 80% of mice from lethal aerosol VEEV exposure [81]. Partial protection was also observed with this vaccine and delivery method in NHP [82]. VEEV DNA vaccination by PMED prevented viremia in two out of three NHP after aerosol VEEV challenge. Fever, lymphopenia, and clinical signs of disease were also reduced in vaccinated animals. In efforts to improve their VEEV DNA vaccine, Dupuy et al., utilized gene-optimization methods and tested an alternative delivery method [83]. Codonoptimization was applied to the VEEV DNA vaccine to reflect codon usage in humans, and the construct was altered to remove unwanted cis-acting RNA motifs. The resulting vaccine plasmid (VEEVco), containing the E3-E2-6K-E1 coding region of VEEV TrD, was tested for increased immunogenicity and/or protective efficacy in several animal models. BALB/c mice were given three vaccinations with VEEVco or control VEEVwt (parental, wild type, un-optimized VEEV DNA vaccine), by i.m. EP. At low doses of vaccine, VEEVco produced a more robust anti-VEEV antibody response in immunized mice compared to VEEVwt. VEEVco also elicited a greater neutralizing antibody response at all doses tested. To assess efficacy, mice were vaccinated twice with VEEVco by i.m. EP, and challenged by aerosol VEEV TrD (>1000 LD50). All VEEVco vaccinated mice survived challenge and showed no signs of disease. Efficacy was also investigated with this optimized DNA vaccine NHPs [83]. Cynomolgus macaques were vaccinated twice with VEEVco by i.m. EP. After aerosol challenge with VEEV TrD, no viremia was detected in vaccinated animals. Fever, lymphopenia, and clinical signs of disease were present, but reduced, in VEEVco-vaccinated NHPs, compared to controls. Additional experiments demonstrate a T-cell response to the vaccine in mice, and longlived (> 6 months) neutralizing antibodies in rabbits.
Directed molecular evolution (i.e. gene shuffling) has generated and identified improved vaccine protein antigens with increased immunogenicity and cross-reactivity. Dupuy el al., applied this technique toward the generation of a multi-agent DNA vaccine for VEEV, EEEV and WEEV [84]. In vitro DNA recombination was performed using cloned E1 and E2 genes from VEEV IAB, VEEV IE, Mucambo virus, EEEV, and WEEV. Recombination events occurred at regions of sequence homology, or at sites engineered for forced crossover recombination. This procedure produced a plasmid library of alphavirus E1 and E2 protein variants, subsequently tested as DNA vaccines in mice. In vitro and in vivo screening procedures identified DNA vaccines with increased immunogenicity and cross reactivity. Select plasmid DNA vaccines elicited a greater antibody response to VEEV IAB compared to the parental, unrecombined VEEV IAB plasmid. Antibodies elicited by this recombined vaccine also cross-reacted with other alphaviruses. In efficacy studies, mice were immunized with parental or recombined DNA vaccines by PMED. After the second and third vaccination, two of the recombined plasmids produced an anti-VEEV antibody response comparable to TC-83 vaccination, along with an enhanced neutralizing antibody response. These plasmids protected 90-100% of mice from lethal aerosol VEEV infection (compared to 80% protection with parental DNA vaccine). Given the observed cross-reactivity for VEEV, EEEV, and WEEV, of the serum antibody from animals vaccinated with the recombined DNA vaccines, it will be interesting to see if protection is observed against alphaviruses other than VEEV. Such an approach could provide an alternative to administering several separate vaccines to protect against these related viruses.
Increased performance of VEEV DNA vaccines can be achieved with a prime-boost immunization strategy [85]. The VEEV gene sequence from RAd-VEEV#3 [65] was used to generate a plasmid for use as a DNA vaccine against VEEV. BALB/c mice were vaccinated with three doses of VEEV DNA vaccine by PMED at two week intervals. The mice were then boosted with Ad-VEEV#3 or not boosted. The Ad vaccine boost elicited a greater VEEV-specific antibody response, and neutralizing antibody response, compared to DNA vaccine alone, or Ad-VEEV#3 alone. The prime-boost regimen also significantly enhanced protection against lethal aerosol VEEV TrD challenge. Further studies are needed to determine the extent of enhanced efficacy that an Ad vaccine boost might achieve after fewer than three initial DNA vaccinations.
An expression plasmid (pVHX-6) containing the 26S subgenomic region of WEEV strain 71V-1658 has been investigated as a DNA vaccine [86]. This plasmid expresses both E1 and E2 proteins of WEEV. BALB/c mice were immunized with four doses of pVHX-6, or empty plasmid control, via Helios Gene Gun i.d. delivery. Mice were given two doses of pVHX-6 on each of two vaccination days, (total of 4 vaccine doses), two weeks apart and challenged two weeks after second set of doses. Immunized mice were 100% protected from i.n. challenge with homologous WEEV. Significant but incomplete protection was observed after challenge with WEEV Fleming or CBA87 strains. With this immunization strategy, an anti-E1 or E2 antibody response was not detected. However, a T-cell response was generated. Additional WEEV DNA vaccines, expressing different portions of the 26S subgenomic region, have been investigated [15]. In comparison to plasmid pVHX-6, constructs lacking the capsid protein coding region, or constructs expressing only E1 or only E2 proteins were studied. Mice were administered three doses of DNA vaccine, two weeks apart, i.d., by gene gun. Notably, the DNA vaccine expressing E1 (p6K-E1) provided complete protection against homologous WEEV, while the DNA vaccine expressing E2 alone provided no protection. However, DNA vaccine p6K-E1 was less effective than pVHX-6 when challenged with a high virulence heterologous WEEV strain.
Subunit vaccines
E1 and E2 glycoproteins have been investigated as subunit vaccines for alphaviruses, and have shown variable efficacy. The E1 or E2 coding sequence from WEEV strain 71V-1658 were cloned and expressed in E. coli [87,88]. The recombinant proteins were immunogenic, producing both antibody and cell-mediated responses in mice, and were recognized by immune serum from mice immunized with inactivated WEEV. However, i.m. immunization with recombinant E1 or E2 proteins formulated with TiterMax Gold adjuvant provided only slight or no protection against homologous or heterologous WEEV virus challenge. In contrast, one study indicated that the structural proteins from VEEV are immunogenic and effective vaccine antigens in mice [89]. Recombinant baculoviruses were utilized to express various regions of the structural gene region of VEEV in Sf9 insect cells. Lysates from these cells, containing VEEV structural gene expression products, were used to immunize BALB/c mice. Lysates containing baculovirusexpressed E1 and E2, or E1 alone provided 100% protection against i.p. challenge with VEEV TrD. This study suggests that purified VEEV E1 or E2 proteins would be effective VEEV vaccine immunogens. Differences in the platforms concerning expression and purity of WEEV and VEEV immunogens could explain the different outcomes of these studies.
Peptides derived from VEEV E2 protein are also immunogenic and protective when administered to mice [90]. BALB/c or NIH Swiss mice were immunized s.c. with free peptides, with or without adjuvant. Anti-peptide and anti-VEEV antibodies were elicited in response to vaccination, although neutralizing antibodies were not detected prior to challenge. Two peptides provided significant protection against i.p. challenge with VEEV TrD in BALB/c and NIH Swiss mice.
Conclusions and Future Directions
Diverse vaccine platforms have proven efficacious in animal models for the prevention of VEEV, EEEV, and WEEV infections. Several of these have been successfully used to develop vaccines for other virus infections, and have passed important safety hurdles in phase I clinical trials. DNA vaccines offer many advantages, though they are often less immunogenic and require more vaccinations or specialized delivery systems (i.e., electroporation) for effective protection, compared to other vaccine platforms.VRPs offer great promise, but are in the early stages of development for alphaviruses. Live-attenuated virus vaccines are often the most immunogenic and efficacious, though safety concerns could limit their licensure. Recent studies of inactivated vaccine candidates indicate that next-generation inactivation methodologies may provide greater protection than those utilized in the early 1960s. This strategy could have an advantage given that a number of FDA-approved vaccines are based on this methodology. Virus-like particles (VLPs) are also likely to emerge as promising alphavirus vaccine candidates. A recent report describes the production and characterization of Chikungunya virus (CHIKV) VLPs [91]. Immunization with CHIKV VLPs produces a neutralizing antibody response in mice and NHPs, and prevents viremia in NHP after subsequent challenge with CHIKV. This strategy will likely be tested in the future as a vaccine strategy for VEEV, EEEV, and WEEV. To date, many of the alphavirus vaccine candidates are immunogenic and efficacious in peripherally challenged mouse models of infection. Additional testing in inhalational models of infection is necessary to determine which platform is the most efficacious as well as licensable. A major hurdle for vaccines against biowarfare agents will be FDA approval. The benefit to risk ratio is inherently low and licensure will require the use of the animal rule. One aspect of alphavirus immunity that remains unanswered is the correlate of protection by which vaccines should be measured for determination of the best vaccine candidate. It is clear that neutralizing and nonneutraling antibodies as well as T cells can aid in the protection against lethal alphavirus infection. Protection in immunocompetent individuals will likely be provided by a multi-armed immune response involving both humoral and cell-mediated immunity. One arm of the immune response may need to dominate depending upon the route of challenge. For example, neutralizing antibody levels correlate with protection following a subcutaneous challenge; yet do not always correlate with protection against an aerosol challenge. It is possible that a T cell and/or a mucosal immune response will be important for protection against lethal disease following inhalation of alphaviruses. The studies reviewed here suggest that the correlate of protection will not be a single solution but different vaccine candidates may have different mechanisms of protection based on the candidate itself, the formulation, route of vaccination, and route of infection. Therefore, the best vaccine candidate ultimately may depend upon the type of infection one is trying to protect against.
Acknowledgements
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. KBS is appointed to the Postgraduate Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and USAMRMC. PJG vaccine research is supported by funding from the Defense Threat Reduction Agency Projects H.H.0003_07_RD_B and 1.1C0040_09_RD_B.
References
- Strauss JH, Strauss EG (1994) The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58: 491-562.
- Reichert E, Clase A, Bacetty A, Larsen J (2009) Alphavirus antiviral drug development: scientific gap analysis and prospective research areas. Biosecur Bioterror 7: 413-427.
- Bernstein BJ (1987) The birth of the U.S. biological-warfare program. Sci Am 256: 116-121.
- Kortepeter MG, Cieslak TJ, Eitzen EM (2001) Bioterrorism. J Environ Health 63: 21-24.
- Barber TL, Walton TE, Lewis KJ (1978) Efficacy of trivalent inactivated encephalomyelitis virus vaccine in horses. Am J Vet Res 39: 621-625.
- Hoke CH (2005) History of U.S. military contributions to the study of viral encephalitis. Mil Med 170: 92-105.
- Burke DS, Ramsburg HH, Edelman R (1977) Persistence in humans of antibody to subtypes of Venezuelan equine encephalomyelitis (VEE) virus after immunization with attenuated (TC-83) VEE virus vaccine. J Infect Dis 136: 354-359.
- Bartelloni PJ, McKinney RW, Duffy TP, Cole FE (1970) An inactivated eastern equine encephalomyelitis vaccine propagated in chick-embryo cell culture. II. Clinical and serologic responses in man. Am J Trop Med Hyg 19: 123-126.
- Liljestrom P, Garoff H (1991) Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol 65: 147-54.
- Moehring JM, Inocencio NM, Robertson BJ, Moehring TJ ( 1993) Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem 268: 2590-2594.
- Dubuisson J, Rice CM (1993) Sindbis virus attachment: isolation and characterization of mutants with impaired binding to vertebrate cells. J Virol 67: 3363-3374.
- Strauss EG, Stec DS, Schmaljohn AL, Strauss JH (1991) Identification of antigenically important domains in the glycoproteins of Sindbis virus by analysis of antibody escape variants. J Virol 65: 4654-4664.
- Zheng Y, Sanchez-San Martin C, Qin ZL, Kielian M (2011) The Domain I-Domain III Linker Plays an Important Role in the Fusogenic Conformational Change of the Alphavirus Membrane Fusion Protein. J Virol 85:6334-6342.
- Kielian M (1995) Membrane fusion and the alphavirus life cycle. Adv Virus Res 45: 113-151.
- Gauci PJ, Wu JQ, Rayner GA, Barabe ND, Nagata LP, et al. (2010) Identification of Western equine encephalitis virus structural proteins that confer protection after DNA vaccination. Clin Vaccine Immunol 17: 176-179.
- Swayze RD, Bhogal HS, Barabe ND, McLaws LJ, Wu JQ (2011) Envelope protein E1 as vaccine target for western equine encephalitis virus. Vaccine 29: 813-820.
- Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (2005) Virus Taxonomy:Eighth Report of the International Committee on Taxonomy of Viruses: Elsevier Academic Press.
- Powers AM, Oberste MS, Brault AC, Rico-Hesse R, Schmura SM, et al. (1997) Repeated emergence of epidemic/epizootic Venezuelan equine encephalitis from a single genotype of enzootic subtype ID virus. J Virol 71: 6697-6705.
- Quiroz E, Aguilar PV, Cisneros J, Tesh RB, Weaver SC (2009) Venezuelan equine encephalitis in Panama: fatal endemic disease and genetic diversity of etiologic viral strains. PLoS Negl Trop Dis 3: e472.
- Vilcarromero S, Aguilar PV, Halsey ES, Laguna-Torres VA, Razuri H, et al. (2010) Venezuelan equine encephalitis and 2 human deaths, Peru. Emerg Infect Dis 16: 553-556.
- Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, et al. (2004) Risk of occupationally acquired illnesses from biological threat agents in unvaccinated laboratory workers. Biosecur Bioterror 2: 281-293.
- Dietz WH, Jr., Peralta PH, Johnson KM (1979) Ten clinical cases of human infection with venezuelan equine encephalomyelitis virus, subtype I-D. Am J Trop Med Hyg 28: 329-334.
- Arrigo NC, Adams AP, Weaver SC (2010) Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 84: 1014-1025.
- Lundstrom JO, Pfeffer M (2010) Phylogeographic structure and evolutionary history of Sindbis virus. Vector Borne Zoonotic Dis 10: 889-907.
- Walton TE (1992) Arboviral encephalomyelitides of livestock in the western hemisphere. J Am Vet Med Assoc 200: 1385-1389.
- Weaver SC, Rico-Hesse R, Scott TW (1992) Genetic diversity and slow rates of evolution in New World alphaviruses. Curr Top Microbiol Immunol 176: 99-117.
- Berge TO, Banks IS, Tigertt WD (1961) Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea-pig hart cells. Am J Hyg 73: 209-218.
- Calisher CH, Maness KS (1975) Laboratory studies of Venezuelan equine encephalitis virus in equines, Texas, 1971. J Clin Microbiol 2: 198-205.
- Ludwig GV, Turell MJ, Vogel P, Kondig JP, Kell WK, et al. (2001) Comparative neurovirulence of attenuated and non-attenuated strains of Venezuelan equine encephalitis virus in mice. Am J Trop Med Hyg 64: 49-55.
- Edelman R, Ascher MS, Oster CN, Ramsburg HH, Cole FE, et al. (1979) Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84). J Infect Dis 140: 708-715.
- Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, et al. (2003) Recombinant sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol 77: 9278-9286.
- Paessler S, Ni H, Petrakova O, Fayzulin RZ, Yun N, et al. (2006) Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J Virol 80: 2784-2796.
- Atasheva S, Wang E, Adams AP, Plante KS, Ni S, et al. (2009) Chimeric alphavirus vaccine candidates protect mice from intranasal challenge with western equine encephalitis virus. Vaccine 27: 4309-4319.
- Kenney JL, Adams AP, Weaver SC (2010) Transmission potential of two chimeric western equine encephalitis vaccine candidates in Culex tarsalis. Am J Trop Med Hyg 82: 354-359.
- Wang E, Petrakova O, Adams AP, Aguilar PV, Kang W, et al. (2007) Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine 25: 7573-7581.
- Arrigo NC, Watts DM, Frolov I, Weaver SC (2008) Experimental infection of Aedes sollicitans and Aedes taeniorhynchus with two chimeric Sindbis/Eastern equine encephalitis virus vaccine candidates. Am J Trop Med Hyg 78: 93-97.
- Ozden S, Lucas-Hourani M, Ceccaldi PE, Basak A, Valentine M, et al. (2008) Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: impairment of the maturation of the E2 surface glycoprotein. J Biol Chem 283: 21899-21908.
- Zhang X, Fugere M, Day R, Kielian M (2003) Furin processing and proteolytic activation of Semliki Forest virus. J Virol 77: 2981-2389.
- de Curtis I, Simons K (1988) Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proceedings of the National Academy of Sciences of the United States of America 85: 8052-8056.
- Mayne JT, Rice CM, Strauss EG, Hunkapiller MW, Strauss JH (1984) Biochemical studies of the maturation of the small Sindbis virus glycoprotein E3. Virology 134: 338-357.
- Turell MJ, O'Guinn ML, Parker MD (2003) Limited potential for mosquito transmission of genetically engineered, live-attenuated western equine encephalitis virus vaccine candidates. Am J Trop Med Hyg 68: 218-221.
- Davis NL, Brown KW, Greenwald GF, Zajac AJ, Zacny VL, et al. (1995) Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology 212: 102-110.
- Hart MK, Caswell-Stephan K, Bakken R, Tammariello R, Pratt W, et al. (2000) Improved mucosal protection against Venezuelan equine encephalitis virus is induced by the molecularly defined, live-attenuated V3526 vaccine candidate. Vaccine 18: 3067-3075.
- Hart MK, Lind C, Bakken R, Robertson M, Tammariello R, et al. (2001) Onset and duration of protective immunity to IA/IB and IE strains of Venezuelan equine encephalitis virus in vaccinated mice. Vaccine 20: 616-622.
- Pratt WD, Davis NL, Johnston RE, Smith JF (2003) Genetically engineered, live attenuated vaccines for Venezuelan equine encephalitis: testing in animal models. Vaccine 21: 3854-3862.
- Fine DL, Roberts BA, Teehee ML, Terpening SJ, Kelly CL, et al. (2007) Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine 25: 1868-1876.
- Fine DL, Roberts BA, Terpening SJ, Mott J, Vasconcelos D, et al. (2008) Neurovirulence evaluation of Venezuelan equine encephalitis (VEE) vaccine candidate V3526 in nonhuman primates. Vaccine 26: 3497-3506.
- Turell MJ, Parker MD (2008) Protection of hamsters by Venezuelan equine encephalitis virus candidate vaccine V3526 against lethal challenge by mosquito bite and intraperitoneal injection. Am J Trop Med Hyg 78: 328-332.
- Fine DL, Jenkins E, Martin SS, Glass P, Parker MD, et al. (2010) A multisystem approach for development and evaluation of inactivated vaccines for Venezuelan equine encephalitis virus (VEEV). J Virol Methods 163: 424-432.
- Martin SS, Bakken RR, Lind CM, Garcia P, Jenkins E, et al. (2010) Comparison of the immunological responses and efficacy of gammairradiated V3526 vaccine formulations against subcutaneous and aerosol challenge with Venezuelan equine encephalitis virus subtype IAB. Vaccine 28: 1031-1040.
- Martin SS, Bakken RR, Lind CM, Garcia P, Jenkins E, et al. (2010) Evaluation of formalin inactivated V3526 virus with adjuvant as a next generation vaccine candidate for Venezuelan equine encephalitis virus. Vaccine 28: 3143-3151.
- Sharma A, Gupta P, Glass PJ, Parker MD, Maheshwari RK (2011) Safety and protective efficacy of INA-inactivated Venezuelan equine encephalitis virus: implication in vaccine development. Vaccine 29: 953-959.
- Raviv Y, Viard M, Bess JW, Chertova E, Blumenthal R (2005) Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope. J Virol 79: 12394-12400.
- Warfield KL, Swenson DL, Olinger GG, Kalina WV, Viard M, et al. (2007) Ebola virus inactivation with preservation of antigenic and structural integrity by a photoinducible alkylating agent. J Infect Dis 196 :S276-S283.
- Lundstrom K (2003) Alphavirus vectors for vaccine production and gene therapy. Expert Rev Vaccines 2: 447-459.
- Barnett SW, Burke B, Sun Y, Kan E, Legg H, et al. (2010) Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol 84: 5975-5985.
- Pan CH, Greer CE, Hauer D, Legg HS, Lee EY, et al. (2010) A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J Virol 84: 3798-3807.
- Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, et al. (2000) Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19: 142-153.
- Draper SJ, Heeney JL (2010) Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 8: 62-73.
- Lasaro MO, Ertl HC (2009) New insights on adenovirus as vaccine vectors. Mol Ther 17: 1333-1339.
- Khanam S, Pilankatta R, Khanna N, Swaminathan S (2009) An adenovirus type 5 (AdV5) vector encoding an envelope domain III-based tetravalent antigen elicits immune responses against all four dengue viruses in the presence of prior AdV5 immunity. Vaccine 27: 6011-6021.
- Li P, Zheng QS, Wang Q, Li Y, Wang EX, et al. (2008) Immune responses of recombinant adenoviruses expressing immunodominant epitopes against Japanese encephalitis virus. Vaccine 26: 5802-5807.
- Gray G, Buchbinder S, Duerr A (2010) Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS 5: 357-361.
- Vemula SV, Mittal SK (2010) Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther 10: 1469-1487.
- Phillpotts RJ, O'Brien L, Appleton RE, Carr S, Bennett A (2005) Intranasal immunisation with defective adenovirus serotype 5 expressing the Venezuelan equine encephalitis virus E2 glycoprotein protects against airborne challenge with virulent virus. Vaccine 23: 1615-1623.
- Perkins SD, Williams AJ, O'Brien LM, Laws TR, Phillpotts RJ (2008) CpG used as an adjuvant for an adenovirus-based Venezuelan equine encephalitis virus vaccine increases the immune response to the vector, but not to the transgene product. Viral Immunol 21: 451-458.
- O'Brien L, Perkins S, Williams A, Eastaugh L, Phelps A, et al. (2009) Alpha interferon as an adenovirus-vectored vaccine adjuvant and antiviral in Venezuelan equine encephalitis virus infection. J Gen Virol 90: 874-882.
- Williams AJ, O'Brien LM, Phillpotts RJ, Perkins SD (2009) Improved efficacy of a gene optimised adenovirus-based vaccine for venezuelan equine encephalitis virus. Virol J 6: 118.
- Wu JQ, Barabe ND, Chau D, Wong C, Rayner GR, et al. (2007) Complete protection of mice against a lethal dose challenge of western equine encephalitis virus after immunization with an adenovirus-vectored vaccine. Vaccine 25: 4368-4375.
- Barabe ND, Rayner GA, Christopher ME, Nagata LP, Wu JQ (2007) Singledose, fast-acting vaccine candidate against western equine encephalitis virus completely protects mice from intranasal challenge with different strains of the virus. Vaccine 25: 6271-6276.
- Rosas CT, Paessler S, Ni H, Osterrieder N (2008) Protection of mice by equine herpesvirus type 1 based experimental vaccine against lethal Venezuelan equine encephalitis virus infection in the absence of neutralizing antibodies. Am J Trop Med Hyg 78: 83-92.
- Grosfeld H, Velan B, Leitner M, Cohen S, Lustig S, et al. (1989) Semliki Forest virus E2 envelope epitopes induce a nonneutralizing humoral response which protects mice against lethal challenge. J Virol 63: 3416-3422.
- Hunt AR, Short WA, Johnson AJ, Bolin RA, Roehrig JT (1991) Synthetic peptides of the E2 glycoprotein of Venezuelan equine encephalomyelitis virus. II. Antibody to the amino terminus protects animals by limiting viral replication. Virology 185: 281-290.
- Brooke CB, Deming DJ, Whitmore AC, White LJ, Johnston RE (2010) T cells facilitate recovery from Venezuelan equine encephalitis virus-induced encephalomyelitis in the absence of antibody. J Virol 84: 4556-4568.
- Paessler S, Yun NE, Judy BM, Dziuba N, Zacks MA, et al. (2007) Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology 367: 307-323.
- Yun NE, Peng BH, Bertke AS, Borisevich V, Smith JK, et al. (2009) CD4+ T cells provide protection against acute lethal encephalitis caused by Venezuelan equine encephalitis virus. Vaccine 27: 4064-4073.
- Kinney RM, Esposito JJ, Mathews JH, Johnson BJ, Roehrig JT, et al. (1988) Recombinant vaccinia virus/Venezuelan equine encephalitis (VEE) virus protects mice from peripheral VEE virus challenge. J Virol 62: 4697-4702.
- Mathews JH, Kinney RM, Roehrig JT, Barrett AD, Trent DW (1994) Murine T-helper cell immune response to recombinant vaccinia-Venezuelan equine encephalitis virus. Vaccine 12: 620-624.
- Bennett AM, Lescott T, Phillpotts RJ (1998) Improved protection against Venezuelan equine encephalitis by genetic engineering of a recombinant vaccinia virus. Viral Immunol 11: 109-117.
- Phillpotts RJ, Lescott TL, Jacobs SC (2000) Vaccinia virus recombinants encoding the truncated structural gene region of Venezuelan equine encephalitis virus (VEEV) give solid protection against peripheral challenge but only partial protection against airborne challenge with virulent VEEV. Acta Virol 44: 233-239.
- Riemenschneider J, Garrison A, Geisbert J, Jahrling P, Hevey M, et al. (2003) Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine 21: 4071-4080.
- Dupuy LC, Richards MJ, Reed DS, Schmal john CS (2010) Immunogenicity and protective efficacy of a DNA vaccine against Venezuelan equine encephalitis virus aerosol challenge in nonhuman primates. Vaccine 28: 7345-7350.
- Dupuy LC, Richards MJ, Ellefsen B, Chau L, Luxembourg A, et al. (2011) A DNA vaccine for venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin Vaccine Immunol 18: 707-716.
- Dupuy LC, Locher CP, Paidhungat M, Richards MJ, Lind CM, et al. (2009) Directed molecular evolution improves the immunogenicity and protective efficacy of a Venezuelan equine encephalitis virus DNA vaccine. Vaccine 27: 4152-4160.
- Perkins SD, O'Brien LM, Phillpotts RJ (2006) Boosting with an adenovirusbased vaccine improves protective efficacy against Venezuelan equine encephalitis virus following DNA vaccination. Vaccine 24: 3440-3445.
- Nagata LP, Hu WG, Masri SA, Rayner GA, Schmaltz FL, et al. (2005) Efficacy of DNA vaccination against western equine encephalitis virus infection. Vaccine 23: 2280-2283.
- Das D, Gares SL, Nagata LP, Suresh MR (2004) Evaluation of a Western Equine Encephalitis recombinant E1 protein for protective immunity and diagnostics. Antiviral Res 64: 85-92.
- Das D, Nagata LP, Suresh MR (2007) Immunological evaluation of Escherichia coli expressed E2 protein of Western equine encephalitis virus. Virus Res 128: 26-33.
- Hodgson LA, Ludwig GV, Smith JF (1999) Expression, processing, and immunogenicity of the structural proteins of Venezuelan equine encephalitis virus from recombinant baculovirus vectors. Vaccine 17: 1151-1160.
- Hunt AR, Johnson AJ, Roehrig JT (1990) Synthetic peptides of Venezuelan equine encephalomyelitis virus E2 glycoprotein I. Immunogenic analysis and identification of a protective peptide. Virology 179: 701-711.
- Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, et al. (2010) A viruslike particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med 16: 334-338.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 17096
- [From(publication date):
specialissue-2011 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12330
- PDF downloads : 4766